2,2-Dimethyl-5-[3-(4-methylphenyl)-2-propenylidene]-1,3-dioxane-4,6-dione, C
16H
16O
4, crystallizes in the triclinic space group
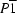
, with two molecules in the asymmetric unit. These molecules and a centrosymmetrically related pair, linked together by weak C-H

O hydrogen bonds, form a tetramer. 5-[3-(4-Chlorophenyl)-2-propenylidene]-2,2-dimethyl-1,3-dioxane-4,6-dione, C
15H
13ClO
4, also crystallizes in the triclinic space group
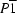
, with one molecule in the asymmetric unit. Centrosymmetrically related molecules are linked together by weak C-H

O hydrogen bonds to form dimers which are further linked by yet another pair of centrosymmetrically related C-H

O hydrogen bonds to form a tube which runs parallel to the
a axis.
Supporting information
CCDC references: 180167; 180168
Preparation of 5-(3-(4-methylphenyl)-2-propenylidene]-2,2-dimethyl-[1,3]
dioxane-4,6-dione (3a). A solution of 4-methylbenzaldehyde, (1a), (2.0 mmoles)
and Meldrum's acid, (2), (4.0 mmoles) in ethoxyethanol (5 ml) with catalytic
amounts of triethylamine was refluxed during 30 minutes (TLC control), the
resulting precipitate was filtered, washed with ethanol, dried and purified by
silica gel chromatography with chloroform. Orange crystals suitable of (3a)
for X-ray diffraction were prepared by diffusion using ethyl acetate and
hexane. Mp 401 K, yield 65%.
Preparation of 5-(3-(4-chlorophenyl)-2-propenylidene]-2,2-dimethyl-[1,3]
dioxane-4,6-dione (3 b). A solution of 4-chlorobenzaldehyde, (1 b), (2.0
mmoles) and Meldrum's acid, (2), (4.0 mmoles) in ethoxyethanol (5 ml) with
catalytic amounts of triethylamine was refluxed during 30 minutes (TLC
control), the resulting precipitate was filtered, washed with ethanol, dried
and purified by silica gel chromatography with chloroform. Orange crystals
suitable of (3 b) for X-ray diffraction were prepared by diffusion using ethyl
acetate and hexane. Mp 455 K, yield 70%.
Molecules (3a & 3 b) crystallized in the triclinic system; space group P-1
assumed and confirmed by the analysis. H atoms were treated as riding atoms
with C—H 0.95 to 0.98 Å. Methyl hydrogen were calculated a six half H
atoms at the corners of a regular hexagon. Examination of the structure with
PLATON (Spek, 2001) showed that there were no solvent accessible voids
in the crystal lattice.
For both compounds, data collection: Kappa-CCD server software (Nonius, 1997). Cell refinement: DENZO-SMN (Otwinowski & Minor, 1997) for (3a); Kappa-CCD server software (Nonius, 1997) for (3b). Data reduction: DENZO-SMN (Otwinowski & Minor, 1997) for (3a); Kappa-CCD server software (Nonius, 1997) for (3b). For both compounds, program(s) used to solve structure: SHELXS97 (Sheldrick, 1997); program(s) used to refine structure: SHELXL97 (Sheldrick, 1997). Molecular graphics: ORTEP (Johnson, 1976), PLATON (Spek, 2001) for (3a); NRCVAX96, ORTEP (Johnson, 1976), PLATON (Spek, 2001) for (3b). For both compounds, software used to prepare material for publication: SHELXL97 and WORDPERFECT macro PRPKAPPA (Ferguson, 2000).
(3a) 5-(3-(4-methylphenyl)-2-propenylidene]-2,2-dimethyl-[1,3] dioxane-4,6-dione
top
Crystal data top
| C16H16O4 | Z = 4 |
| Mr = 272.29 | F(000) = 576 |
| Triclinic, P1 | Dx = 1.288 Mg m−3 |
| Hall symbol: -P 1 | Melting point: 401 K |
| a = 11.2891 (3) Å | Mo Kα radiation, λ = 0.71073 Å |
| b = 11.4128 (4) Å | Cell parameters from 6331 reflections |
| c = 13.4504 (5) Å | θ = 2.9–27.5° |
| α = 91.217 (2)° | µ = 0.09 mm−1 |
| β = 114.640 (3)° | T = 120 K |
| γ = 113.7590 (15)° | Lath, colourless |
| V = 1403.88 (8) Å3 | 0.15 × 0.15 × 0.1 mm |
Data collection top
Kappa-CCD
diffractometer | 6331 independent reflections |
| Radiation source: fine-focus sealed X-ray tube | 3359 reflections with I > 2σ(I) |
| Graphite monochromator | Rint = 0.080 |
| ϕ scans and ω scans with κ offsets | θmax = 27.5°, θmin = 2.9° |
Absorption correction: multi-scan
DENZO-SMN (Otwinowski & Minor, 1997) | h = −14→14 |
| Tmin = 0.991, Tmax = 0.999 | k = −14→14 |
| 21945 measured reflections | l = −17→17 |
Refinement top
| Refinement on F2 | Primary atom site location: structure-invariant direct methods |
| Least-squares matrix: full | Secondary atom site location: difference Fourier map |
| R[F2 > 2σ(F2)] = 0.062 | Hydrogen site location: inferred from neighbouring sites |
| wR(F2) = 0.165 | H-atom parameters constrained |
| S = 0.96 | w = 1/[σ2(Fo2) + (0.0809P)2]
where P = (Fo2 + 2Fc2)/3 |
| 6331 reflections | (Δ/σ)max < 0.001 |
| 367 parameters | Δρmax = 0.25 e Å−3 |
| 0 restraints | Δρmin = −0.50 e Å−3 |
Crystal data top
| C16H16O4 | γ = 113.7590 (15)° |
| Mr = 272.29 | V = 1403.88 (8) Å3 |
| Triclinic, P1 | Z = 4 |
| a = 11.2891 (3) Å | Mo Kα radiation |
| b = 11.4128 (4) Å | µ = 0.09 mm−1 |
| c = 13.4504 (5) Å | T = 120 K |
| α = 91.217 (2)° | 0.15 × 0.15 × 0.1 mm |
| β = 114.640 (3)° | |
Data collection top
Kappa-CCD
diffractometer | 6331 independent reflections |
Absorption correction: multi-scan
DENZO-SMN (Otwinowski & Minor, 1997) | 3359 reflections with I > 2σ(I) |
| Tmin = 0.991, Tmax = 0.999 | Rint = 0.080 |
| 21945 measured reflections | |
Refinement top
| R[F2 > 2σ(F2)] = 0.062 | 0 restraints |
| wR(F2) = 0.165 | H-atom parameters constrained |
| S = 0.96 | Δρmax = 0.25 e Å−3 |
| 6331 reflections | Δρmin = −0.50 e Å−3 |
| 367 parameters | |
Special details top
Experimental. The program DENZO-SMN (Otwinowski & Minor, 1997) uses a scaling algorithm
(Fox & Holmes, 1966) which effectively corrects for absorption effects. High
redundancy data were used in the scaling program hence the 'multi-scan' code
word was used. No transmission coefficients are available from the program
(only scale factors for each frame). The scale factors in the experimental
table are calculated from the 'size' command in the SHELXL97 input
file. |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2) top| | x | y | z | Uiso*/Ueq | |
| O11 | 0.25428 (17) | 0.07135 (15) | 0.57136 (13) | 0.0359 (4) | |
| C12 | 0.3651 (2) | 0.0548 (2) | 0.55603 (18) | 0.0327 (6) | |
| C121 | 0.4221 (3) | 0.1634 (2) | 0.5019 (2) | 0.0412 (6) | |
| C122 | 0.4809 (3) | 0.0560 (2) | 0.66668 (18) | 0.0426 (6) | |
| O13 | 0.30146 (16) | −0.06671 (14) | 0.47684 (11) | 0.0320 (4) | |
| C14 | 0.2119 (2) | −0.1784 (2) | 0.49390 (17) | 0.0288 (5) | |
| O14 | 0.19023 (17) | −0.28312 (16) | 0.44886 (12) | 0.0378 (4) | |
| C15 | 0.1453 (2) | −0.1604 (2) | 0.56262 (16) | 0.0275 (5) | |
| C16 | 0.1585 (2) | −0.0295 (2) | 0.59303 (17) | 0.0304 (5) | |
| O16 | 0.08966 (18) | −0.00424 (16) | 0.63185 (13) | 0.0412 (4) | |
| C17 | 0.0672 (2) | −0.2682 (2) | 0.59001 (16) | 0.0297 (5) | |
| C18 | −0.0094 (2) | −0.2771 (2) | 0.65323 (17) | 0.0302 (5) | |
| C19 | −0.0818 (2) | −0.3936 (2) | 0.67307 (16) | 0.0294 (5) | |
| C191 | −0.1641 (2) | −0.4159 (2) | 0.73519 (16) | 0.0261 (5) | |
| C192 | −0.1893 (2) | −0.3190 (2) | 0.77604 (17) | 0.0289 (5) | |
| C193 | −0.2664 (2) | −0.3448 (2) | 0.83681 (16) | 0.0301 (5) | |
| C194 | −0.3221 (2) | −0.4687 (2) | 0.85977 (16) | 0.0275 (5) | |
| C91 | −0.4076 (3) | −0.4976 (2) | 0.92450 (18) | 0.0358 (6) | |
| C195 | −0.2986 (2) | −0.5650 (2) | 0.81877 (17) | 0.0307 (5) | |
| C196 | −0.2230 (2) | −0.5409 (2) | 0.75661 (17) | 0.0298 (5) | |
| O21 | −0.31404 (16) | −0.06950 (14) | 0.89824 (12) | 0.0312 (4) | |
| C22 | −0.2865 (3) | −0.0430 (2) | 1.01284 (18) | 0.0325 (6) | |
| C221 | −0.4148 (3) | −0.1473 (2) | 1.0201 (2) | 0.0500 (7) | |
| C222 | −0.1419 (3) | −0.0383 (2) | 1.09044 (18) | 0.0384 (6) | |
| O23 | −0.28717 (17) | 0.08017 (14) | 1.03894 (12) | 0.0344 (4) | |
| C24 | −0.2123 (2) | 0.1856 (2) | 1.00726 (17) | 0.0282 (5) | |
| O24 | −0.19663 (17) | 0.29170 (15) | 1.04345 (12) | 0.0363 (4) | |
| C25 | −0.1633 (2) | 0.1603 (2) | 0.92736 (16) | 0.0262 (5) | |
| C26 | −0.2246 (2) | 0.0246 (2) | 0.86595 (17) | 0.0282 (5) | |
| O26 | −0.20926 (17) | −0.00941 (15) | 0.78898 (12) | 0.0383 (4) | |
| C27 | −0.0675 (2) | 0.2672 (2) | 0.91029 (16) | 0.0279 (5) | |
| C28 | −0.0046 (2) | 0.2744 (2) | 0.83730 (17) | 0.0278 (5) | |
| C29 | 0.0899 (2) | 0.3936 (2) | 0.83520 (17) | 0.0289 (5) | |
| C291 | 0.1671 (2) | 0.4208 (2) | 0.76888 (16) | 0.0272 (5) | |
| C292 | 0.1696 (2) | 0.3235 (2) | 0.70598 (17) | 0.0304 (5) | |
| C293 | 0.2447 (2) | 0.3554 (2) | 0.64403 (17) | 0.0326 (5) | |
| C294 | 0.3214 (2) | 0.4858 (2) | 0.64154 (17) | 0.0330 (6) | |
| C92 | 0.4030 (3) | 0.5200 (3) | 0.57376 (19) | 0.0442 (7) | |
| C295 | 0.3203 (2) | 0.5827 (2) | 0.70421 (18) | 0.0349 (6) | |
| C296 | 0.2457 (2) | 0.5523 (2) | 0.76738 (17) | 0.0305 (5) | |
| H12A | 0.4569 | 0.2485 | 0.5492 | 0.062* | |
| H12B | 0.3436 | 0.1520 | 0.4279 | 0.062* | |
| H12C | 0.5031 | 0.1604 | 0.4935 | 0.062* | |
| H12D | 0.4368 | −0.0182 | 0.6960 | 0.064* | |
| H12E | 0.5230 | 0.1385 | 0.7205 | 0.064* | |
| H12F | 0.5581 | 0.0486 | 0.6552 | 0.064* | |
| H17 | 0.0632 | −0.3482 | 0.5635 | 0.036* | |
| H18 | −0.0096 | −0.2004 | 0.6818 | 0.036* | |
| H19 | −0.0783 | −0.4678 | 0.6434 | 0.035* | |
| H192 | −0.1526 | −0.2339 | 0.7617 | 0.035* | |
| H193 | −0.2818 | −0.2772 | 0.8635 | 0.036* | |
| H91A | −0.3994 | −0.5691 | 0.9614 | 0.054* | |
| H91B | −0.5106 | −0.5238 | 0.8730 | 0.054* | |
| H91C | −0.3687 | −0.4187 | 0.9814 | 0.054* | |
| H195 | −0.3353 | −0.6498 | 0.8336 | 0.037* | |
| H196 | −0.2108 | −0.6098 | 0.7281 | 0.036* | |
| H22A | −0.4230 | −0.2340 | 0.9991 | 0.075* | |
| H22B | −0.5045 | −0.1431 | 0.9687 | 0.075* | |
| H22C | −0.4007 | −0.1327 | 1.0971 | 0.075* | |
| H22D | −0.0633 | 0.0341 | 1.0835 | 0.058* | |
| H22E | −0.1423 | −0.1216 | 1.0703 | 0.058* | |
| H22F | −0.1259 | −0.0244 | 1.1681 | 0.058* | |
| H27 | −0.0385 | 0.3495 | 0.9540 | 0.033* | |
| H28 | −0.0283 | 0.1966 | 0.7904 | 0.033* | |
| H29 | 0.1072 | 0.4677 | 0.8828 | 0.035* | |
| H292 | 0.1186 | 0.2339 | 0.7059 | 0.037* | |
| H293 | 0.2444 | 0.2872 | 0.6021 | 0.039* | |
| H92D | 0.5075 | 0.5509 | 0.6235 | 0.066* | |
| H92E | 0.3882 | 0.5895 | 0.5367 | 0.066* | |
| H92F | 0.3664 | 0.4419 | 0.5169 | 0.066* | |
| H295 | 0.3717 | 0.6721 | 0.7040 | 0.042* | |
| H296 | 0.2477 | 0.6210 | 0.8101 | 0.037* | |
Atomic displacement parameters (Å2) top| | U11 | U22 | U33 | U12 | U13 | U23 |
| O11 | 0.0376 (10) | 0.0269 (9) | 0.0520 (10) | 0.0126 (8) | 0.0304 (8) | 0.0116 (7) |
| C12 | 0.0330 (13) | 0.0243 (13) | 0.0447 (14) | 0.0095 (11) | 0.0249 (11) | 0.0057 (10) |
| C121 | 0.0392 (15) | 0.0342 (15) | 0.0573 (15) | 0.0132 (12) | 0.0317 (13) | 0.0153 (12) |
| C122 | 0.0333 (14) | 0.0381 (16) | 0.0424 (14) | 0.0096 (12) | 0.0120 (12) | 0.0035 (11) |
| O13 | 0.0333 (9) | 0.0273 (9) | 0.0366 (9) | 0.0089 (8) | 0.0220 (7) | 0.0071 (7) |
| C14 | 0.0237 (12) | 0.0302 (14) | 0.0302 (12) | 0.0094 (11) | 0.0132 (10) | 0.0066 (10) |
| O14 | 0.0405 (10) | 0.0286 (10) | 0.0467 (10) | 0.0109 (8) | 0.0272 (8) | 0.0024 (7) |
| C15 | 0.0243 (12) | 0.0275 (13) | 0.0292 (11) | 0.0090 (10) | 0.0139 (10) | 0.0042 (9) |
| C16 | 0.0309 (13) | 0.0308 (14) | 0.0306 (12) | 0.0118 (11) | 0.0174 (10) | 0.0091 (10) |
| O16 | 0.0507 (11) | 0.0340 (10) | 0.0567 (10) | 0.0206 (9) | 0.0386 (9) | 0.0139 (8) |
| C17 | 0.0256 (12) | 0.0277 (13) | 0.0309 (12) | 0.0084 (11) | 0.0128 (10) | 0.0022 (9) |
| C18 | 0.0276 (13) | 0.0284 (14) | 0.0328 (12) | 0.0094 (11) | 0.0158 (10) | 0.0031 (10) |
| C19 | 0.0260 (12) | 0.0274 (14) | 0.0312 (12) | 0.0086 (11) | 0.0138 (10) | 0.0033 (9) |
| C191 | 0.0208 (11) | 0.0254 (13) | 0.0258 (11) | 0.0064 (10) | 0.0095 (9) | 0.0044 (9) |
| C192 | 0.0280 (12) | 0.0201 (13) | 0.0327 (12) | 0.0061 (10) | 0.0137 (10) | 0.0037 (9) |
| C193 | 0.0314 (13) | 0.0256 (13) | 0.0315 (12) | 0.0103 (11) | 0.0158 (10) | 0.0006 (9) |
| C194 | 0.0247 (12) | 0.0270 (13) | 0.0256 (11) | 0.0081 (10) | 0.0107 (9) | 0.0055 (9) |
| C91 | 0.0336 (13) | 0.0397 (15) | 0.0370 (13) | 0.0147 (12) | 0.0205 (11) | 0.0111 (11) |
| C195 | 0.0297 (13) | 0.0242 (13) | 0.0362 (12) | 0.0098 (11) | 0.0159 (10) | 0.0102 (10) |
| C196 | 0.0302 (13) | 0.0243 (13) | 0.0377 (12) | 0.0124 (11) | 0.0181 (10) | 0.0075 (10) |
| O21 | 0.0300 (9) | 0.0217 (9) | 0.0387 (9) | 0.0077 (7) | 0.0174 (7) | 0.0055 (6) |
| C22 | 0.0406 (14) | 0.0232 (13) | 0.0443 (14) | 0.0150 (11) | 0.0281 (12) | 0.0114 (10) |
| C221 | 0.0569 (18) | 0.0269 (15) | 0.0816 (19) | 0.0129 (13) | 0.0510 (16) | 0.0173 (13) |
| C222 | 0.0496 (16) | 0.0335 (15) | 0.0372 (13) | 0.0228 (13) | 0.0206 (12) | 0.0122 (11) |
| O23 | 0.0412 (10) | 0.0221 (9) | 0.0536 (10) | 0.0136 (8) | 0.0343 (8) | 0.0128 (7) |
| C24 | 0.0257 (12) | 0.0250 (14) | 0.0355 (12) | 0.0123 (11) | 0.0148 (10) | 0.0076 (10) |
| O24 | 0.0450 (10) | 0.0239 (10) | 0.0498 (10) | 0.0154 (8) | 0.0305 (8) | 0.0088 (7) |
| C25 | 0.0240 (12) | 0.0238 (13) | 0.0321 (12) | 0.0112 (10) | 0.0138 (10) | 0.0077 (9) |
| C26 | 0.0243 (12) | 0.0264 (13) | 0.0334 (12) | 0.0119 (11) | 0.0127 (10) | 0.0092 (10) |
| O26 | 0.0453 (11) | 0.0326 (10) | 0.0377 (9) | 0.0147 (8) | 0.0231 (8) | 0.0036 (7) |
| C27 | 0.0299 (12) | 0.0254 (13) | 0.0311 (12) | 0.0152 (11) | 0.0139 (10) | 0.0074 (9) |
| C28 | 0.0299 (13) | 0.0274 (13) | 0.0325 (12) | 0.0153 (11) | 0.0177 (10) | 0.0081 (9) |
| C29 | 0.0294 (13) | 0.0288 (14) | 0.0344 (12) | 0.0156 (11) | 0.0174 (10) | 0.0087 (10) |
| C291 | 0.0223 (11) | 0.0274 (13) | 0.0305 (11) | 0.0105 (10) | 0.0118 (9) | 0.0083 (9) |
| C292 | 0.0276 (13) | 0.0255 (13) | 0.0359 (12) | 0.0117 (11) | 0.0131 (10) | 0.0059 (10) |
| C293 | 0.0323 (13) | 0.0368 (15) | 0.0307 (12) | 0.0169 (12) | 0.0154 (10) | 0.0042 (10) |
| C294 | 0.0282 (13) | 0.0428 (16) | 0.0304 (12) | 0.0164 (12) | 0.0151 (10) | 0.0113 (10) |
| C92 | 0.0386 (15) | 0.0576 (18) | 0.0410 (14) | 0.0187 (14) | 0.0253 (12) | 0.0138 (12) |
| C295 | 0.0332 (14) | 0.0302 (14) | 0.0441 (14) | 0.0118 (12) | 0.0226 (11) | 0.0160 (11) |
| C296 | 0.0314 (13) | 0.0257 (13) | 0.0388 (13) | 0.0139 (11) | 0.0193 (11) | 0.0081 (10) |
Geometric parameters (Å, º) top
| O11—C16 | 1.361 (3) | O21—C26 | 1.369 (3) |
| O11—C12 | 1.431 (3) | O21—C22 | 1.438 (2) |
| C12—O13 | 1.439 (3) | C22—O23 | 1.445 (3) |
| C12—C121 | 1.505 (3) | C22—C221 | 1.503 (3) |
| C12—C122 | 1.512 (3) | C22—C222 | 1.504 (3) |
| C121—H12A | 0.9800 | C221—H22A | 0.9800 |
| C121—H12B | 0.9800 | C221—H22B | 0.9800 |
| C121—H12C | 0.9800 | C221—H22C | 0.9800 |
| C122—H12D | 0.9800 | C222—H22D | 0.9800 |
| C122—H12E | 0.9800 | C222—H22E | 0.9800 |
| C122—H12F | 0.9800 | C222—H22F | 0.9800 |
| O13—C14 | 1.366 (3) | O23—C24 | 1.359 (3) |
| C14—O14 | 1.210 (3) | C24—O24 | 1.210 (2) |
| C14—C15 | 1.469 (3) | C24—C25 | 1.469 (3) |
| C15—C17 | 1.358 (3) | C25—C27 | 1.367 (3) |
| C15—C16 | 1.471 (3) | C25—C26 | 1.471 (3) |
| C16—O16 | 1.213 (2) | C26—O26 | 1.200 (2) |
| C17—C18 | 1.424 (3) | C27—C28 | 1.418 (3) |
| C17—H17 | 0.9500 | C27—H27 | 0.9500 |
| C18—C19 | 1.352 (3) | C28—C29 | 1.359 (3) |
| C18—H18 | 0.9500 | C28—H28 | 0.9500 |
| C19—C191 | 1.447 (3) | C29—C291 | 1.447 (3) |
| C19—H19 | 0.9500 | C29—H29 | 0.9500 |
| C191—C192 | 1.401 (3) | C291—C292 | 1.398 (3) |
| C191—C196 | 1.404 (3) | C291—C296 | 1.408 (3) |
| C192—C193 | 1.382 (3) | C292—C293 | 1.379 (3) |
| C192—H192 | 0.9500 | C292—H292 | 0.9500 |
| C193—C194 | 1.398 (3) | C293—C294 | 1.397 (3) |
| C193—H193 | 0.9500 | C293—H293 | 0.9500 |
| C194—C195 | 1.383 (3) | C294—C295 | 1.384 (3) |
| C194—C91 | 1.502 (3) | C294—C92 | 1.503 (3) |
| C91—H91A | 0.9800 | C92—H92D | 0.9800 |
| C91—H91B | 0.9800 | C92—H92E | 0.9800 |
| C91—H91C | 0.9800 | C92—H92F | 0.9800 |
| C195—C196 | 1.386 (3) | C295—C296 | 1.387 (3) |
| C195—H195 | 0.9500 | C295—H295 | 0.9500 |
| C196—H196 | 0.9500 | C296—H296 | 0.9500 |
| | | |
| C16—O11—C12 | 119.10 (17) | C26—O21—C22 | 117.62 (17) |
| O11—C12—O13 | 110.18 (17) | O21—C22—O23 | 109.68 (16) |
| O11—C12—C121 | 105.73 (18) | O21—C22—C221 | 106.72 (19) |
| O13—C12—C121 | 106.45 (17) | O23—C22—C221 | 105.83 (18) |
| O11—C12—C122 | 110.58 (18) | O21—C22—C222 | 109.83 (18) |
| O13—C12—C122 | 109.84 (18) | O23—C22—C222 | 110.53 (18) |
| C121—C12—C122 | 113.9 (2) | C221—C22—C222 | 114.1 (2) |
| C12—C121—H12A | 109.5 | C22—C221—H22A | 109.5 |
| C12—C121—H12B | 109.5 | C22—C221—H22B | 109.5 |
| H12A—C121—H12B | 109.5 | H22A—C221—H22B | 109.5 |
| C12—C121—H12C | 109.5 | C22—C221—H22C | 109.5 |
| H12A—C121—H12C | 109.5 | H22A—C221—H22C | 109.5 |
| H12B—C121—H12C | 109.5 | H22B—C221—H22C | 109.5 |
| C12—C122—H12D | 109.5 | C22—C222—H22D | 109.5 |
| C12—C122—H12E | 109.5 | C22—C222—H22E | 109.5 |
| H12D—C122—H12E | 109.5 | H22D—C222—H22E | 109.5 |
| C12—C122—H12F | 109.5 | C22—C222—H22F | 109.5 |
| H12D—C122—H12F | 109.5 | H22D—C222—H22F | 109.5 |
| H12E—C122—H12F | 109.5 | H22E—C222—H22F | 109.5 |
| C14—O13—C12 | 117.70 (16) | C24—O23—C22 | 118.58 (16) |
| O14—C14—O13 | 118.02 (19) | O24—C24—O23 | 117.51 (19) |
| O14—C14—C15 | 125.5 (2) | O24—C24—C25 | 125.3 (2) |
| O13—C14—C15 | 116.41 (19) | O23—C24—C25 | 117.10 (19) |
| C17—C15—C14 | 117.7 (2) | C27—C25—C24 | 117.0 (2) |
| C17—C15—C16 | 123.0 (2) | C27—C25—C26 | 123.64 (19) |
| C14—C15—C16 | 119.17 (19) | C24—C25—C26 | 119.31 (19) |
| O16—C16—O11 | 117.7 (2) | O26—C26—O21 | 118.2 (2) |
| O16—C16—C15 | 126.4 (2) | O26—C26—C25 | 126.8 (2) |
| O11—C16—C15 | 115.87 (19) | O21—C26—C25 | 114.88 (18) |
| C15—C17—C18 | 128.5 (2) | C25—C27—C28 | 129.9 (2) |
| C15—C17—H17 | 115.7 | C25—C27—H27 | 115.0 |
| C18—C17—H17 | 115.7 | C28—C27—H27 | 115.0 |
| C19—C18—C17 | 120.7 (2) | C29—C28—C27 | 119.3 (2) |
| C19—C18—H18 | 119.6 | C29—C28—H28 | 120.3 |
| C17—C18—H18 | 119.6 | C27—C28—H28 | 120.3 |
| C18—C19—C191 | 126.1 (2) | C28—C29—C291 | 127.6 (2) |
| C18—C19—H19 | 116.9 | C28—C29—H29 | 116.2 |
| C191—C19—H19 | 116.9 | C291—C29—H29 | 116.2 |
| C192—C191—C196 | 117.28 (19) | C292—C291—C296 | 117.4 (2) |
| C192—C191—C19 | 123.6 (2) | C292—C291—C29 | 123.7 (2) |
| C196—C191—C19 | 119.1 (2) | C296—C291—C29 | 118.90 (19) |
| C193—C192—C191 | 121.3 (2) | C293—C292—C291 | 121.1 (2) |
| C193—C192—H192 | 119.4 | C293—C292—H292 | 119.5 |
| C191—C192—H192 | 119.4 | C291—C292—H292 | 119.5 |
| C192—C193—C194 | 121.2 (2) | C292—C293—C294 | 121.5 (2) |
| C192—C193—H193 | 119.4 | C292—C293—H293 | 119.2 |
| C194—C193—H193 | 119.4 | C294—C293—H293 | 119.2 |
| C195—C194—C193 | 117.8 (2) | C295—C294—C293 | 117.7 (2) |
| C195—C194—C91 | 121.0 (2) | C295—C294—C92 | 121.0 (2) |
| C193—C194—C91 | 121.3 (2) | C293—C294—C92 | 121.3 (2) |
| C194—C91—H91A | 109.5 | C294—C92—H92D | 109.5 |
| C194—C91—H91B | 109.5 | C294—C92—H92E | 109.5 |
| H91A—C91—H91B | 109.5 | H92D—C92—H92E | 109.5 |
| C194—C91—H91C | 109.5 | C294—C92—H92F | 109.5 |
| H91A—C91—H91C | 109.5 | H92D—C92—H92F | 109.5 |
| H91B—C91—H91C | 109.5 | H92E—C92—H92F | 109.5 |
| C194—C195—C196 | 121.7 (2) | C294—C295—C296 | 121.5 (2) |
| C194—C195—H195 | 119.2 | C294—C295—H295 | 119.3 |
| C196—C195—H195 | 119.2 | C296—C295—H295 | 119.3 |
| C195—C196—C191 | 120.8 (2) | C295—C296—C291 | 120.8 (2) |
| C195—C196—H196 | 119.6 | C295—C296—H296 | 119.6 |
| C191—C196—H196 | 119.6 | C291—C296—H296 | 119.6 |
| | | |
| C16—O11—C12—O13 | 49.5 (2) | C26—O21—C22—O23 | 55.0 (2) |
| C16—O11—C12—C121 | 164.12 (18) | C26—O21—C22—C221 | 169.22 (18) |
| C16—O11—C12—C122 | −72.1 (2) | C26—O21—C22—C222 | −66.6 (2) |
| O11—C12—O13—C14 | −50.6 (2) | O21—C22—O23—C24 | −46.0 (2) |
| C121—C12—O13—C14 | −164.80 (17) | C221—C22—O23—C24 | −160.77 (19) |
| C122—C12—O13—C14 | 71.4 (2) | C222—C22—O23—C24 | 75.2 (2) |
| C12—O13—C14—O14 | −160.17 (19) | C22—O23—C24—O24 | −169.70 (18) |
| C12—O13—C14—C15 | 22.2 (3) | C22—O23—C24—C25 | 13.2 (3) |
| O14—C14—C15—C17 | 8.7 (3) | O24—C24—C25—C27 | 14.0 (3) |
| O13—C14—C15—C17 | −173.80 (18) | O23—C24—C25—C27 | −169.16 (18) |
| O14—C14—C15—C16 | −167.8 (2) | O24—C24—C25—C26 | −162.6 (2) |
| O13—C14—C15—C16 | 9.6 (3) | O23—C24—C25—C26 | 14.2 (3) |
| C12—O11—C16—O16 | 162.67 (19) | C22—O21—C26—O26 | 154.03 (19) |
| C12—O11—C16—C15 | −19.5 (3) | C22—O21—C26—C25 | −29.3 (2) |
| C17—C15—C16—O16 | −9.9 (3) | C27—C25—C26—O26 | −6.2 (3) |
| C14—C15—C16—O16 | 166.5 (2) | C24—C25—C26—O26 | 170.2 (2) |
| C17—C15—C16—O11 | 172.47 (19) | C27—C25—C26—O21 | 177.45 (18) |
| C14—C15—C16—O11 | −11.2 (3) | C24—C25—C26—O21 | −6.2 (3) |
| C14—C15—C17—C18 | −178.75 (19) | C24—C25—C27—C28 | −178.17 (19) |
| C16—C15—C17—C18 | −2.3 (4) | C26—C25—C27—C28 | −1.7 (3) |
| C15—C17—C18—C19 | −179.8 (2) | C25—C27—C28—C29 | −179.7 (2) |
| C17—C18—C19—C191 | −179.61 (19) | C27—C28—C29—C291 | 178.55 (19) |
| C18—C19—C191—C192 | 5.4 (3) | C28—C29—C291—C292 | −8.7 (3) |
| C18—C19—C191—C196 | −174.6 (2) | C28—C29—C291—C296 | 171.8 (2) |
| C196—C191—C192—C193 | 1.2 (3) | C296—C291—C292—C293 | −0.7 (3) |
| C19—C191—C192—C193 | −178.8 (2) | C29—C291—C292—C293 | 179.75 (19) |
| C191—C192—C193—C194 | 0.0 (3) | C291—C292—C293—C294 | 0.0 (3) |
| C192—C193—C194—C195 | −0.5 (3) | C292—C293—C294—C295 | 0.4 (3) |
| C192—C193—C194—C91 | −179.19 (19) | C292—C293—C294—C92 | −179.9 (2) |
| C193—C194—C195—C196 | −0.2 (3) | C293—C294—C295—C296 | −0.1 (3) |
| C91—C194—C195—C196 | 178.43 (19) | C92—C294—C295—C296 | −179.8 (2) |
| C194—C195—C196—C191 | 1.5 (3) | C294—C295—C296—C291 | −0.6 (3) |
| C192—C191—C196—C195 | −2.0 (3) | C292—C291—C296—C295 | 1.0 (3) |
| C19—C191—C196—C195 | 178.00 (19) | C29—C291—C296—C295 | −179.46 (19) |
Hydrogen-bond geometry (Å, º) top
| D—H···A | D—H | H···A | D···A | D—H···A |
| C29—H29···O24i | 0.95 | 2.52 | 3.412 (3) | 156 |
| C193—H193···O21 | 0.95 | 2.60 | 3.531 (3) | 166 |
| C296—H296···O24i | 0.95 | 2.56 | 3.425 (3) | 152 |
| C293—H293···O11 | 0.95 | 2.54 | 3.422 (3) | 154 |
| Symmetry code: (i) −x, −y+1, −z+2. |
(3b) 5-(3-(4-chlorophenyl)-2-propenylidene]-2,2-dimethyl-[1,3] dioxane-4,6-dione
top
Crystal data top
| C15H13ClO4 | Z = 2 |
| Mr = 292.70 | F(000) = 304 |
| Triclinic, P1 | Dx = 1.423 Mg m−3 |
| Hall symbol: -P 1 | Melting point: 455K K |
| a = 7.2648 (2) Å | Mo Kα radiation, λ = 0.71073 Å |
| b = 7.4705 (3) Å | Cell parameters from 3057 reflections |
| c = 13.5855 (6) Å | θ = 3.0–27.5° |
| α = 90.1799 (13)° | µ = 0.29 mm−1 |
| β = 99.8452 (14)° | T = 120 K |
| γ = 109.576 (3)° | Block, yellow |
| V = 683.01 (4) Å3 | 0.15 × 0.10 × 0.10 mm |
Data collection top
Kappa-CCD
diffractometer | 3057 independent reflections |
| Radiation source: fine-focus sealed X-ray tube | 2350 reflections with I > 2σ(I) |
| Graphite monochromator | Rint = 0.077 |
| ϕ scans and ω scans with κ offsets | θmax = 27.5°, θmin = 3.0° |
Absorption correction: multi-scan
DENZO-SMN (Otwinowski & Minor, 1997) | h = −9→8 |
| Tmin = 0.958, Tmax = 0.972 | k = −9→9 |
| 9407 measured reflections | l = −17→17 |
Refinement top
| Refinement on F2 | Primary atom site location: structure-invariant direct methods |
| Least-squares matrix: full | Secondary atom site location: difference Fourier map |
| R[F2 > 2σ(F2)] = 0.053 | Hydrogen site location: inferred from neighbouring sites |
| wR(F2) = 0.153 | H-atom parameters constrained |
| S = 0.98 | w = 1/[σ2(Fo2) + (0.0932P)2 + 0.2802P]
where P = (Fo2 + 2Fc2)/3 |
| 3057 reflections | (Δ/σ)max = 0.001 |
| 183 parameters | Δρmax = 0.62 e Å−3 |
| 0 restraints | Δρmin = −0.66 e Å−3 |
Crystal data top
| C15H13ClO4 | γ = 109.576 (3)° |
| Mr = 292.70 | V = 683.01 (4) Å3 |
| Triclinic, P1 | Z = 2 |
| a = 7.2648 (2) Å | Mo Kα radiation |
| b = 7.4705 (3) Å | µ = 0.29 mm−1 |
| c = 13.5855 (6) Å | T = 120 K |
| α = 90.1799 (13)° | 0.15 × 0.10 × 0.10 mm |
| β = 99.8452 (14)° | |
Data collection top
Kappa-CCD
diffractometer | 3057 independent reflections |
Absorption correction: multi-scan
DENZO-SMN (Otwinowski & Minor, 1997) | 2350 reflections with I > 2σ(I) |
| Tmin = 0.958, Tmax = 0.972 | Rint = 0.077 |
| 9407 measured reflections | |
Refinement top
| R[F2 > 2σ(F2)] = 0.053 | 0 restraints |
| wR(F2) = 0.153 | H-atom parameters constrained |
| S = 0.98 | Δρmax = 0.62 e Å−3 |
| 3057 reflections | Δρmin = −0.66 e Å−3 |
| 183 parameters | |
Special details top
Experimental. The program DENZO-SMN (Otwinowski & Minor, 1997) uses a scaling algorithm
(Fox & Holmes, 1966) which effectively corrects for absorption effects. High
redundancy data were used in the scaling program hence the 'multi-scan' code
word was used. No transmission coefficients are available from the program
(only scale factors for each frame). The scale factors in the experimental
table are calculated from the 'size' command in the SHELXL97 input
file. |
Geometry. Mean-plane data from the final SHELXL97 refinement run:- |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2) top| | x | y | z | Uiso*/Ueq | Occ. (<1) |
| O1 | 1.2361 (2) | 0.8733 (2) | 0.73887 (10) | 0.0205 (3) | |
| C2 | 1.1593 (3) | 0.8931 (3) | 0.63540 (15) | 0.0193 (5) | |
| C21 | 1.3360 (3) | 0.9621 (3) | 0.58476 (17) | 0.0241 (5) | |
| C22 | 1.0071 (3) | 0.7068 (3) | 0.58938 (17) | 0.0250 (5) | |
| O3 | 1.0719 (2) | 1.0403 (2) | 0.63026 (11) | 0.0203 (4) | |
| C4 | 0.9434 (3) | 1.0391 (3) | 0.69179 (15) | 0.0195 (5) | |
| O4 | 0.8406 (2) | 1.1372 (2) | 0.67387 (11) | 0.0259 (4) | |
| C5 | 0.9478 (3) | 0.9239 (3) | 0.78005 (15) | 0.0184 (4) | |
| C6 | 1.1172 (3) | 0.8565 (3) | 0.80810 (15) | 0.0192 (4) | |
| O6 | 1.1626 (2) | 0.7951 (2) | 0.88697 (11) | 0.0270 (4) | |
| C7 | 0.8004 (3) | 0.8958 (3) | 0.83404 (16) | 0.0195 (4) | |
| C8 | 0.7819 (3) | 0.8043 (3) | 0.92632 (16) | 0.0205 (5) | |
| C9 | 0.6261 (3) | 0.7897 (3) | 0.97112 (16) | 0.0194 (4) | |
| C91 | 0.5980 (3) | 0.7101 (3) | 1.06755 (15) | 0.0184 (4) | |
| C92 | 0.7295 (3) | 0.6295 (3) | 1.12205 (16) | 0.0218 (5) | |
| C93 | 0.7025 (3) | 0.5620 (3) | 1.21510 (17) | 0.0227 (5) | |
| C94 | 0.5441 (3) | 0.5750 (3) | 1.25471 (16) | 0.0207 (5) | |
| Cl97 | 0.51673 (8) | 0.49493 (8) | 1.37336 (4) | 0.0283 (2) | |
| C95 | 0.4077 (3) | 0.6503 (3) | 1.20245 (16) | 0.0220 (5) | |
| C96 | 0.4360 (3) | 0.7163 (3) | 1.10875 (16) | 0.0201 (5) | |
| H21A | 1.4067 | 0.8705 | 0.5914 | 0.036* | 0.50 |
| H21B | 1.4252 | 1.0861 | 0.6162 | 0.036* | 0.50 |
| H21C | 1.2916 | 0.9749 | 0.5137 | 0.036* | 0.50 |
| H21D | 1.3423 | 1.0838 | 0.5561 | 0.036* | 0.50 |
| H21E | 1.3238 | 0.8682 | 0.5313 | 0.036* | 0.50 |
| H21F | 1.4574 | 0.9795 | 0.6338 | 0.036* | 0.50 |
| H22A | 1.0659 | 0.6061 | 0.5973 | 0.038* | 0.50 |
| H22B | 0.9644 | 0.7188 | 0.5180 | 0.038* | 0.50 |
| H22C | 0.8921 | 0.6746 | 0.6230 | 0.038* | 0.50 |
| H22D | 0.8824 | 0.7269 | 0.5616 | 0.038* | 0.50 |
| H22E | 0.9839 | 0.6143 | 0.6408 | 0.038* | 0.50 |
| H22F | 1.0562 | 0.6584 | 0.5359 | 0.038* | 0.50 |
| H7 | 0.6968 | 0.9424 | 0.8074 | 0.023* | |
| H8 | 0.8803 | 0.7535 | 0.9561 | 0.025* | |
| H9 | 0.5265 | 0.8350 | 0.9371 | 0.023* | |
| H92 | 0.8385 | 0.6211 | 1.0947 | 0.026* | |
| H93 | 0.7920 | 0.5073 | 1.2514 | 0.027* | |
| H95 | 0.2982 | 0.6563 | 1.2301 | 0.026* | |
| H96 | 0.3434 | 0.7668 | 1.0718 | 0.024* | |
Atomic displacement parameters (Å2) top| | U11 | U22 | U33 | U12 | U13 | U23 |
| O1 | 0.0196 (8) | 0.0316 (8) | 0.0127 (7) | 0.0118 (6) | 0.0035 (6) | 0.0026 (6) |
| C2 | 0.0223 (11) | 0.0269 (11) | 0.0115 (10) | 0.0123 (9) | 0.0024 (8) | 0.0035 (8) |
| C21 | 0.0241 (12) | 0.0324 (13) | 0.0192 (11) | 0.0120 (9) | 0.0084 (9) | 0.0053 (9) |
| C22 | 0.0265 (12) | 0.0284 (12) | 0.0191 (11) | 0.0089 (9) | 0.0022 (9) | −0.0003 (9) |
| O3 | 0.0223 (8) | 0.0278 (8) | 0.0143 (7) | 0.0115 (6) | 0.0065 (6) | 0.0047 (6) |
| C4 | 0.0182 (11) | 0.0247 (11) | 0.0149 (10) | 0.0071 (8) | 0.0016 (8) | 0.0003 (8) |
| O4 | 0.0275 (9) | 0.0386 (10) | 0.0188 (8) | 0.0197 (7) | 0.0058 (6) | 0.0071 (7) |
| C5 | 0.0190 (11) | 0.0220 (11) | 0.0139 (10) | 0.0073 (8) | 0.0016 (8) | 0.0001 (8) |
| C6 | 0.0194 (11) | 0.0254 (11) | 0.0128 (10) | 0.0080 (8) | 0.0024 (8) | 0.0010 (8) |
| O6 | 0.0284 (9) | 0.0424 (10) | 0.0159 (8) | 0.0192 (7) | 0.0043 (6) | 0.0088 (7) |
| C7 | 0.0197 (11) | 0.0221 (11) | 0.0158 (10) | 0.0065 (8) | 0.0021 (8) | −0.0003 (8) |
| C8 | 0.0208 (11) | 0.0236 (11) | 0.0174 (11) | 0.0081 (8) | 0.0031 (8) | 0.0018 (9) |
| C9 | 0.0209 (11) | 0.0216 (11) | 0.0149 (10) | 0.0066 (8) | 0.0023 (8) | 0.0003 (8) |
| C91 | 0.0195 (11) | 0.0178 (10) | 0.0154 (10) | 0.0036 (8) | 0.0024 (8) | −0.0006 (8) |
| C92 | 0.0191 (11) | 0.0280 (12) | 0.0186 (11) | 0.0080 (9) | 0.0045 (8) | 0.0013 (9) |
| C93 | 0.0235 (11) | 0.0239 (11) | 0.0206 (11) | 0.0086 (9) | 0.0026 (9) | 0.0038 (9) |
| C94 | 0.0248 (11) | 0.0210 (11) | 0.0134 (10) | 0.0042 (8) | 0.0029 (8) | 0.0009 (8) |
| Cl97 | 0.0326 (4) | 0.0357 (4) | 0.0169 (3) | 0.0104 (2) | 0.0074 (2) | 0.0084 (2) |
| C95 | 0.0222 (11) | 0.0267 (11) | 0.0175 (11) | 0.0073 (9) | 0.0067 (8) | 0.0003 (9) |
| C96 | 0.0203 (11) | 0.0223 (11) | 0.0187 (11) | 0.0084 (8) | 0.0038 (8) | 0.0014 (8) |
Geometric parameters (Å, º) top
| O1—C6 | 1.361 (2) | C5—C7 | 1.359 (3) |
| O1—C2 | 1.448 (2) | C5—C6 | 1.473 (3) |
| C2—O3 | 1.439 (2) | C6—O6 | 1.205 (2) |
| C2—C21 | 1.500 (3) | C7—C8 | 1.435 (3) |
| C2—C22 | 1.509 (3) | C7—H7 | 0.9500 |
| C21—H21A | 0.9800 | C8—C9 | 1.348 (3) |
| C21—H21B | 0.9800 | C8—H8 | 0.9500 |
| C21—H21C | 0.9800 | C9—C91 | 1.458 (3) |
| C21—H21D | 0.9800 | C9—H9 | 0.9500 |
| C21—H21E | 0.9800 | C91—C96 | 1.401 (3) |
| C21—H21F | 0.9800 | C91—C92 | 1.404 (3) |
| C22—H22A | 0.9800 | C92—C93 | 1.383 (3) |
| C22—H22B | 0.9800 | C92—H92 | 0.9500 |
| C22—H22C | 0.9800 | C93—C94 | 1.382 (3) |
| C22—H22D | 0.9800 | C93—H93 | 0.9500 |
| C22—H22E | 0.9800 | C94—C95 | 1.392 (3) |
| C22—H22F | 0.9800 | C94—Cl97 | 1.741 (2) |
| O3—C4 | 1.353 (2) | C95—C96 | 1.390 (3) |
| C4—O4 | 1.208 (3) | C95—H95 | 0.9500 |
| C4—C5 | 1.480 (3) | C96—H96 | 0.9500 |
| | | |
| C6—O1—C2 | 119.05 (16) | C2—C22—H22F | 109.5 |
| O3—C2—O1 | 109.57 (16) | H22A—C22—H22F | 56.3 |
| O3—C2—C21 | 106.71 (17) | H22B—C22—H22F | 56.3 |
| O1—C2—C21 | 106.16 (17) | H22C—C22—H22F | 141.1 |
| O3—C2—C22 | 110.29 (17) | H22D—C22—H22F | 109.5 |
| O1—C2—C22 | 110.17 (17) | H22E—C22—H22F | 109.5 |
| C21—C2—C22 | 113.78 (18) | C4—O3—C2 | 118.97 (16) |
| C2—C21—H21A | 109.5 | O4—C4—O3 | 118.49 (19) |
| C2—C21—H21B | 109.5 | O4—C4—C5 | 124.7 (2) |
| H21A—C21—H21B | 109.5 | O3—C4—C5 | 116.73 (17) |
| C2—C21—H21C | 109.5 | C7—C5—C6 | 123.64 (19) |
| H21A—C21—H21C | 109.5 | C7—C5—C4 | 117.82 (18) |
| H21B—C21—H21C | 109.5 | C6—C5—C4 | 118.42 (18) |
| C2—C21—H21D | 109.5 | O6—C6—O1 | 117.95 (19) |
| H21A—C21—H21D | 141.1 | O6—C6—C5 | 126.09 (19) |
| H21B—C21—H21D | 56.3 | O1—C6—C5 | 115.91 (17) |
| H21C—C21—H21D | 56.3 | C5—C7—C8 | 128.0 (2) |
| C2—C21—H21E | 109.5 | C5—C7—H7 | 116.0 |
| H21A—C21—H21E | 56.3 | C8—C7—H7 | 116.0 |
| H21B—C21—H21E | 141.1 | C9—C8—C7 | 120.59 (19) |
| H21C—C21—H21E | 56.3 | C9—C8—H8 | 119.7 |
| H21D—C21—H21E | 109.5 | C7—C8—H8 | 119.7 |
| C2—C21—H21F | 109.5 | C8—C9—C91 | 125.4 (2) |
| H21A—C21—H21F | 56.3 | C8—C9—H9 | 117.3 |
| H21B—C21—H21F | 56.3 | C91—C9—H9 | 117.3 |
| H21C—C21—H21F | 141.1 | C96—C91—C92 | 118.28 (19) |
| H21D—C21—H21F | 109.5 | C96—C91—C9 | 119.28 (19) |
| H21E—C21—H21F | 109.5 | C92—C91—C9 | 122.4 (2) |
| C2—C22—H22A | 109.5 | C93—C92—C91 | 120.9 (2) |
| C2—C22—H22B | 109.5 | C93—C92—H92 | 119.6 |
| H22A—C22—H22B | 109.5 | C91—C92—H92 | 119.6 |
| C2—C22—H22C | 109.5 | C94—C93—C92 | 119.3 (2) |
| H22A—C22—H22C | 109.5 | C94—C93—H93 | 120.3 |
| H22B—C22—H22C | 109.5 | C92—C93—H93 | 120.3 |
| C2—C22—H22D | 109.5 | C93—C94—C95 | 121.8 (2) |
| H22A—C22—H22D | 141.1 | C93—C94—Cl97 | 118.56 (17) |
| H22B—C22—H22D | 56.3 | C95—C94—Cl97 | 119.68 (17) |
| H22C—C22—H22D | 56.3 | C96—C95—C94 | 118.2 (2) |
| C2—C22—H22E | 109.5 | C96—C95—H95 | 120.9 |
| H22A—C22—H22E | 56.3 | C94—C95—H95 | 120.9 |
| H22B—C22—H22E | 141.1 | C95—C96—C91 | 121.5 (2) |
| H22C—C22—H22E | 56.3 | C95—C96—H96 | 119.3 |
| H22D—C22—H22E | 109.5 | C91—C96—H96 | 119.3 |
| | | |
| C6—O1—C2—O3 | 49.7 (2) | C4—C5—C6—O1 | −12.8 (3) |
| C6—O1—C2—C21 | 164.55 (17) | C4—C5—C7—C8 | −174.20 (19) |
| C6—O1—C2—C22 | −71.8 (2) | C6—C5—C7—C8 | 1.9 (4) |
| O1—C2—O3—C4 | −48.4 (2) | C5—C7—C8—C9 | 179.3 (2) |
| C21—C2—O3—C4 | −162.88 (17) | C7—C8—C9—C91 | −176.20 (19) |
| C22—C2—O3—C4 | 73.1 (2) | C8—C9—C91—C92 | −3.5 (3) |
| C2—O3—C4—O4 | −165.13 (19) | C8—C9—C91—C96 | 175.4 (2) |
| C2—O3—C4—C5 | 18.1 (3) | C96—C91—C92—C93 | −1.5 (3) |
| O4—C4—C5—C7 | 13.6 (3) | C9—C91—C92—C93 | 177.4 (2) |
| O3—C4—C5—C7 | −169.81 (18) | C91—C92—C93—C94 | −0.2 (3) |
| O4—C4—C5—C6 | −162.7 (2) | C92—C93—C94—C95 | 1.5 (3) |
| O3—C4—C5—C6 | 13.9 (3) | C92—C93—C94—Cl97 | −178.33 (17) |
| C2—O1—C6—O6 | 162.40 (19) | C93—C94—C95—C96 | −1.1 (3) |
| C2—O1—C6—C5 | −20.1 (3) | Cl97—C94—C95—C96 | 178.77 (16) |
| C7—C5—C6—O6 | −11.5 (4) | C94—C95—C96—C91 | −0.7 (3) |
| C4—C5—C6—O6 | 164.5 (2) | C92—C91—C96—C95 | 2.0 (3) |
| C7—C5—C6—O1 | 171.21 (19) | C9—C91—C96—C95 | −177.03 (19) |
Hydrogen-bond geometry (Å, º) top
| D—H···A | D—H | H···A | D···A | D—H···A |
| C9—H9···O6i | 0.95 | 2.53 | 3.380 (3) | 149 |
| C95—H95···O4ii | 0.95 | 2.56 | 3.396 (3) | 146 |
| Symmetry codes: (i) x−1, y, z; (ii) −x+1, −y+2, −z+2. |
Experimental details
| | (3a) | (3b) |
| Crystal data |
| Chemical formula | C16H16O4 | C15H13ClO4 |
| Mr | 272.29 | 292.70 |
| Crystal system, space group | Triclinic, P1 | Triclinic, P1 |
| Temperature (K) | 120 | 120 |
| a, b, c (Å) | 11.2891 (3), 11.4128 (4), 13.4504 (5) | 7.2648 (2), 7.4705 (3), 13.5855 (6) |
| α, β, γ (°) | 91.217 (2), 114.640 (3), 113.7590 (15) | 90.1799 (13), 99.8452 (14), 109.576 (3) |
| V (Å3) | 1403.88 (8) | 683.01 (4) |
| Z | 4 | 2 |
| Radiation type | Mo Kα | Mo Kα |
| µ (mm−1) | 0.09 | 0.29 |
| Crystal size (mm) | 0.15 × 0.15 × 0.1 | 0.15 × 0.10 × 0.10 |
| |
| Data collection |
| Diffractometer | Kappa-CCD
diffractometer | Kappa-CCD
diffractometer |
| Absorption correction | Multi-scan
DENZO-SMN (Otwinowski & Minor, 1997) | Multi-scan
DENZO-SMN (Otwinowski & Minor, 1997) |
| Tmin, Tmax | 0.991, 0.999 | 0.958, 0.972 |
No. of measured, independent and
observed [I > 2σ(I)] reflections | 21945, 6331, 3359 | 9407, 3057, 2350 |
| Rint | 0.080 | 0.077 |
| (sin θ/λ)max (Å−1) | 0.649 | 0.651 |
| |
| Refinement |
| R[F2 > 2σ(F2)], wR(F2), S | 0.062, 0.165, 0.96 | 0.053, 0.153, 0.98 |
| No. of reflections | 6331 | 3057 |
| No. of parameters | 367 | 183 |
| H-atom treatment | H-atom parameters constrained | H-atom parameters constrained |
| Δρmax, Δρmin (e Å−3) | 0.25, −0.50 | 0.62, −0.66 |
Selected torsion angles (º) for (3a) top| C14—C15—C17—C18 | −178.75 (19) | C24—C25—C27—C28 | −178.17 (19) |
| C16—C15—C17—C18 | −2.3 (4) | C26—C25—C27—C28 | −1.7 (3) |
| C15—C17—C18—C19 | −179.8 (2) | C25—C27—C28—C29 | −179.7 (2) |
| C17—C18—C19—C191 | −179.61 (19) | C27—C28—C29—C291 | 178.55 (19) |
| C18—C19—C191—C192 | 5.4 (3) | C28—C29—C291—C292 | −8.7 (3) |
| C18—C19—C191—C196 | −174.6 (2) | C28—C29—C291—C296 | 171.8 (2) |
Hydrogen-bond geometry (Å, º) for (3a) top
| D—H···A | D—H | H···A | D···A | D—H···A |
| C29—H29···O24i | 0.95 | 2.52 | 3.412 (3) | 156 |
| C193—H193···O21 | 0.95 | 2.60 | 3.531 (3) | 166 |
| C296—H296···O24i | 0.95 | 2.56 | 3.425 (3) | 152 |
| C293—H293···O11 | 0.95 | 2.54 | 3.422 (3) | 154 |
| Symmetry code: (i) −x, −y+1, −z+2. |
Selected torsion angles (º) for (3b) top| C4—C5—C7—C8 | −174.20 (19) | C7—C8—C9—C91 | −176.20 (19) |
| C6—C5—C7—C8 | 1.9 (4) | C8—C9—C91—C92 | −3.5 (3) |
| C5—C7—C8—C9 | 179.3 (2) | C8—C9—C91—C96 | 175.4 (2) |
Hydrogen-bond geometry (Å, º) for (3b) top
| D—H···A | D—H | H···A | D···A | D—H···A |
| C9—H9···O6i | 0.95 | 2.53 | 3.380 (3) | 149 |
| C95—H95···O4ii | 0.95 | 2.56 | 3.396 (3) | 146 |
| Symmetry codes: (i) x−1, y, z; (ii) −x+1, −y+2, −z+2. |
 O hydrogen bonds, form a tetramer. 5-[3-(4-Chlorophenyl)-2-propenylidene]-2,2-dimethyl-1,3-dioxane-4,6-dione, C15H13ClO4, also crystallizes in the triclinic space group
O hydrogen bonds, form a tetramer. 5-[3-(4-Chlorophenyl)-2-propenylidene]-2,2-dimethyl-1,3-dioxane-4,6-dione, C15H13ClO4, also crystallizes in the triclinic space group  O hydrogen bonds to form dimers which are further linked by yet another pair of centrosymmetrically related C-H
O hydrogen bonds to form dimers which are further linked by yet another pair of centrosymmetrically related C-H O hydrogen bonds to form a tube which runs parallel to the a axis.
O hydrogen bonds to form a tube which runs parallel to the a axis.
 journal menu
journal menu








![[Figure 1]](http://journals.iucr.org/c/issues/2002/01/00/sk1522/sk1522fig1thm.gif)
![[Figure 2]](http://journals.iucr.org/c/issues/2002/01/00/sk1522/sk1522fig2thm.gif)
![[Figure 3]](http://journals.iucr.org/c/issues/2002/01/00/sk1522/sk1522fig3thm.gif)
![[Figure 4]](http://journals.iucr.org/c/issues/2002/01/00/sk1522/sk1522fig4thm.gif)




Meldrum's acid and its derivatives serve as good synthetic equivalents, most often in preparation of arylidene condensation products, which have received little attention as intermediates in heterocyclic synthesis. The condensation of Meldrum's acid arylidene derivatives with heterocyclic monoamines to afford pyridines has been reported(Morales et al., 1996, Quiroga et al., 1997, Quiroga et al., 1998, Quiroga et al., 1999, Rodríguez et al., 1996 and Rodríguez et al., 1997) or with α,β-unsaturated or dicarbonyl compounds to coumarins (Margaretha, 1972)
Here, we report an attempt to prepare triazolopyrimidines or triazolopyridazine using such condensation reaction with Meldrum's acid, 1,2-diaminotriazole and benzaldehyde. The final product was surprisingly the 3-(phenyl)-2-propenylidene derivative of Meldrum's acid, which comes from condensation of two molecules of Meldrum's acid with one of benzaldehyde. A further reaction using only the Meldrum's acid and benzaldehydes also produced that compound.
Both the title compounds crystallize in spacegroup P-1, in the case of compound (3a) with two molecules in the asymmetric unit which was chosen such that it formed a centrosymmetric dimer, see below. A view of the molecules of (3a) are shown in Figures 1a and 1 b. A view of molecule (3 b) is shown in Figure 2. The numbering system is chosen so that it is consistent for all three molecules.
The bonds and angles for the three molecules discussed in this study show no major differences except for the torsion angles involving the junction of the phenyl group and the propylidene chain. These difference are probably related to the differences in supramolecular structures found for the compounds. Selected torsion angles are given in Tables 1 for (3a) and 3 for (3 b).
The supramolecular structures of each compound are different. In compound (3a) the two molecules in the asymmetric unit are linked together head-to-tail via the C193–H193···O21 and C293–H293···O11 weak hydrogen bonds to form a dimer comprising an R22(20) ring, (Bernstein et al., 1995). These dimers are further linked together by two centrosymmetrically related pairs of weak C–H···O hydrogen bonds in which O24 at (-x, 1 - y, 2 - z) is an acceptor for H29 and H296 thus forming an R12(6) ring, (Bernstein et al., 1995). Hence a discrete tetramer is formed by the molecules in the asymmetric unit dimer and the dimer related to it by the centre of symmetry at (0, 1/2, 1), Figure 3.
In compound (3 b) in which there is only one molecule in the centrosymmetric unit, the C95–H95···O4(1 - x,2 - y,2 - z) weak hydrogen bond links the molecules into a centrosymmetric dimer centred on (1/2, 1.0, 1.0) giving an R22(20) ring, (Bernstein et al., 1995). In addition the molecules are linked by the C9–H9···O6(-1 + x,y,z) weak hydrogen bond to form C(7) chain running parallel to the a axis. Operation of the centre of symmetry at (0,1,1) produces an R44(28) ring, (Bernstein et al., 1995), resulting from a combination of centrosymmetrically related pairs of the C95–H95···O4 and C9–H9···O6 hydrogen bonds, Figure 4. The result is a tube like three-dimensional ribbon which runs parallel to the a axis.
Details of the hydrogen bonding is given in Tables 2 for (3a) and and (3 b).
There is also a short Cl97···Cl97(1 - x,1 - y,3 - z) contact of 3.4927 (8) Å in (3 b). A preliminary search of the CSD (Allen & Kennard, 1993) gave 1544 hits for short Cl–Cl contacts less than the van der Waals contact of 3.6 Å, for Cl atoms attached to C.