1.3 Å resolution crystal structures of the cyanide, nitric oxide and hydroxylamine complexes of Arthromyces ramosus peroxidase (ARP), a class II peroxidase belonging to the plant peroxidase superfamily, have been determined. Anisotropic temperature factors were introduced for all non-H atoms of these complexes using SHELX-97 and stereochemical constraints were applied to the protein, protoporphyrin and sugar moieties, but not to the coordination geometries to the haem iron. These refinements identified multiple conformations for several side chains and revised the side-chain conformations of several residues. Little difference was observed in the structures of the polypeptides, haem and sugar moieties and in the coordinations to two calcium ions in these complexes. Characteristic coordination geometries of each ligand to the haem iron were observed. CN− binds to the haem iron in a tilt mode (Fe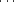 C—N = 170°), whereas NO and hydroxylamine bind in bent modes (Fe
C—N = 170°), whereas NO and hydroxylamine bind in bent modes (Fe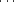 N—O = 125° and Fe
N—O = 125° and Fe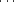 NH2—OH = 111°). CN− is directed toward the distal histidine (His56) and forms a hydrogen bond with the N
NH2—OH = 111°). CN− is directed toward the distal histidine (His56) and forms a hydrogen bond with the N![[epsilon]](/logos/entities/epsiv_rmgif.gif) atom, whereas NO and hydroxylamine are directed away from His56. The Fe atoms of ARP–CN and ARP–NO, in which the haem irons are both in low-spin states, are approximately in the pyrrole N plane, whereas the iron in native ARP, which is in a five-coordinated high-spin state, deviates markedly from the plane.
atom, whereas NO and hydroxylamine are directed away from His56. The Fe atoms of ARP–CN and ARP–NO, in which the haem irons are both in low-spin states, are approximately in the pyrrole N plane, whereas the iron in native ARP, which is in a five-coordinated high-spin state, deviates markedly from the plane.
![[epsilon]](/logos/entities/epsiv_rmgif.gif) atom, whereas NO and hydroxylamine are directed away from His56. The Fe atoms of ARP–CN and ARP–NO, in which the haem irons are both in low-spin states, are approximately in the pyrrole N plane, whereas the iron in native ARP, which is in a five-coordinated high-spin state, deviates markedly from the plane.
atom, whereas NO and hydroxylamine are directed away from His56. The Fe atoms of ARP–CN and ARP–NO, in which the haem irons are both in low-spin states, are approximately in the pyrrole N plane, whereas the iron in native ARP, which is in a five-coordinated high-spin state, deviates markedly from the plane.Supporting information
PDB references: CN-bound form of Arthromyces ramosus peroxidase, 2e39, r2e39sf; NO-bound form, 2e3a, r2e3asf; HA-bound form, 2e3b, r2e3bsf

 journal menu
journal menu











