The space group of the title compound, C
7H
7BO
3, previously reported to be
P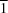
, is properly
Cc. There is no disorder of the formyl group or in the H atoms of the B(OH)
2 group. Molecules lie on approximate twofold axes and are related by approximate centers, which relate all but the formyl O atom and boronic acid H atoms. The B-O distances are 1.363 (2) and 1.370 (2) Å.
Supporting information
CCDC reference: 179270
The preparation of the title compound has been previously described by Feulner,
et al. (1990). In our preparation, compound (I) (300 mg, 0.448 mmol),
27 ml of dimethylsulfoxide (DMSO), and 3 ml of water were mixed and heated for
5 days at 493 K in a sealed tube. The reaction mixture was cooled, filtered,
and DMSO/H2O was removed in vacuo. The yellowish substance is washed
with ethyl acetate (EtOAc) and upon drying afforded colorless crystals of the
title compound (.210 g) in 72% yield. Diffraction-quality crystals of (II)
were grown by evaporation of an EtOAc solution.
Systematic absences indicate space groups Cc or C2/c.
Although intensity statistics suggest C2/c (|E2-1| = 0.992),
the noncentrosymmetric Cc proved correct. The absolute structure could
not be determined. The coordinates of hydroxyl H atoms were refined. Other H
atoms were treated as riding in idealized positions, with C—H distances of
0.95 Å. Displacement parameters for H were assigned as Uiso =
1.2Ueq of the attached atom (1.5 for OH).
Data collection: COLLECT (Nonius, 2000); cell refinement: DENZO and SCALEPAK; data reduction: DENZO and SCALEPAK (Otwinowski & Minor, 1997); program(s) used to solve structure: SIR97 (Altomare et al., 1999); program(s) used to refine structure: SHELXL97 (Sheldrick, 1997); molecular graphics: ORTEP-3 (Farrugia, 1997); software used to prepare material for publication: SHELXL97.
4-formylphenylboronic acid
top
Crystal data top
| C7H7BO3 | F(000) = 312 |
| Mr = 149.94 | Dx = 1.441 Mg m−3 |
| Monoclinic, Cc | Mo Kα radiation, λ = 0.71073 Å |
| a = 11.1238 (3) Å | Cell parameters from 1107 reflections |
| b = 9.8718 (3) Å | θ = 2.5–32.0° |
| c = 7.1988 (2) Å | µ = 0.11 mm−1 |
| β = 119.071 (2)° | T = 120 K |
| V = 690.92 (3) Å3 | Lath fragment, colorless |
| Z = 4 | 0.37 × 0.25 × 0.22 mm |
Data collection top
Nonius KappaCCD (with Oxford Cryostream)
diffractometer | 1110 reflections with I > 2σ(I) |
| Radiation source: fine-focus sealed tube | Rint = 0.021 |
| Graphite monochromator | θmax = 32.0°, θmin = 2.5° |
| ω scans with κ offsets | h = −15→16 |
| 4518 measured reflections | k = −14→14 |
| 1192 independent reflections | l = −10→10 |
Refinement top
| Refinement on F2 | Primary atom site location: structure-invariant direct methods |
| Least-squares matrix: full | Secondary atom site location: difference Fourier map |
| R[F2 > 2σ(F2)] = 0.038 | Hydrogen site location: inferred from neighbouring sites |
| wR(F2) = 0.104 | H atoms treated by a mixture of independent and constrained refinement |
| S = 1.07 | w = 1/[σ2(Fo2) + (0.0689P)2 + 0.1001P]
where P = (Fo2 + 2Fc2)/3 |
| 1192 reflections | (Δ/σ)max < 0.001 |
| 106 parameters | Δρmax = 0.44 e Å−3 |
| 2 restraints | Δρmin = −0.22 e Å−3 |
Crystal data top
| C7H7BO3 | V = 690.92 (3) Å3 |
| Mr = 149.94 | Z = 4 |
| Monoclinic, Cc | Mo Kα radiation |
| a = 11.1238 (3) Å | µ = 0.11 mm−1 |
| b = 9.8718 (3) Å | T = 120 K |
| c = 7.1988 (2) Å | 0.37 × 0.25 × 0.22 mm |
| β = 119.071 (2)° | |
Data collection top
Nonius KappaCCD (with Oxford Cryostream)
diffractometer | 1110 reflections with I > 2σ(I) |
| 4518 measured reflections | Rint = 0.021 |
| 1192 independent reflections | |
Refinement top
| R[F2 > 2σ(F2)] = 0.038 | 2 restraints |
| wR(F2) = 0.104 | H atoms treated by a mixture of independent and constrained refinement |
| S = 1.07 | Δρmax = 0.44 e Å−3 |
| 1192 reflections | Δρmin = −0.22 e Å−3 |
| 106 parameters | |
Special details top
Geometry. All e.s.d.'s (except the e.s.d. in the dihedral angle between two l.s. planes)
are estimated using the full covariance matrix. The cell e.s.d.'s are taken
into account individually in the estimation of e.s.d.'s in distances, angles
and torsion angles; correlations between e.s.d.'s in cell parameters are only
used when they are defined by crystal symmetry. An approximate (isotropic)
treatment of cell e.s.d.'s is used for estimating e.s.d.'s involving l.s.
planes. |
Refinement. Refinement of F2 against ALL reflections. The weighted R-factor
wR and goodness of fit S are based on F2, conventional
R-factors R are based on F, with F set to zero for
negative F2. The threshold expression of F2 >
σ(F2) is used only for calculating R-factors(gt) etc.
and is not relevant to the choice of reflections for refinement.
R-factors based on F2 are statistically about twice as large
as those based on F, and R- factors based on ALL data will be
even larger. |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2) top| | x | y | z | Uiso*/Ueq | |
| O1 | 0.40379 (15) | 0.06712 (11) | 0.7216 (3) | 0.0272 (3) | |
| O2 | 0.37961 (12) | 0.79497 (11) | 0.6533 (2) | 0.0201 (3) | |
| H2O | 0.390 (3) | 0.882 (3) | 0.664 (4) | 0.030* | |
| O3 | 0.61978 (12) | 0.80567 (12) | 0.8461 (2) | 0.0211 (3) | |
| H3O | 0.694 (3) | 0.765 (3) | 0.919 (4) | 0.032* | |
| B1 | 0.5035 (2) | 0.72881 (13) | 0.7509 (4) | 0.0157 (3) | |
| C1 | 0.5029 (2) | 0.56955 (10) | 0.7475 (3) | 0.0145 (2) | |
| C2 | 0.61997 (15) | 0.49629 (15) | 0.7820 (3) | 0.0166 (3) | |
| H2 | 0.7007 | 0.5436 | 0.8061 | 0.020* | |
| C3 | 0.61955 (16) | 0.35504 (14) | 0.7813 (3) | 0.0170 (3) | |
| H3 | 0.6993 | 0.3066 | 0.8039 | 0.020* | |
| C4 | 0.5014 (2) | 0.28490 (12) | 0.7473 (4) | 0.0163 (2) | |
| C5 | 0.38315 (15) | 0.35606 (15) | 0.7117 (2) | 0.0166 (3) | |
| H5 | 0.3027 | 0.3085 | 0.6882 | 0.020* | |
| C6 | 0.38449 (15) | 0.49707 (15) | 0.7111 (3) | 0.0160 (3) | |
| H6 | 0.3040 | 0.5453 | 0.6856 | 0.019* | |
| C7 | 0.5022 (2) | 0.13559 (13) | 0.7469 (4) | 0.0205 (3) | |
| H7 | 0.5831 | 0.0904 | 0.7673 | 0.025* | |
Atomic displacement parameters (Å2) top| | U11 | U22 | U33 | U12 | U13 | U23 |
| O1 | 0.0225 (6) | 0.0138 (5) | 0.0429 (7) | −0.0026 (4) | 0.0140 (5) | −0.0017 (5) |
| O2 | 0.0149 (5) | 0.0105 (5) | 0.0293 (7) | 0.0002 (4) | 0.0062 (5) | 0.0001 (4) |
| O3 | 0.0133 (5) | 0.0141 (5) | 0.0287 (7) | −0.0010 (4) | 0.0044 (5) | 0.0002 (4) |
| B1 | 0.0148 (5) | 0.0125 (6) | 0.0178 (5) | −0.0008 (7) | 0.0064 (4) | −0.0001 (8) |
| C1 | 0.0141 (5) | 0.0115 (5) | 0.0160 (5) | 0.0006 (6) | 0.0059 (4) | 0.0009 (7) |
| C2 | 0.0139 (7) | 0.0149 (6) | 0.0195 (7) | −0.0016 (5) | 0.0071 (6) | −0.0005 (6) |
| C3 | 0.0142 (7) | 0.0138 (6) | 0.0212 (8) | 0.0024 (5) | 0.0073 (7) | −0.0004 (5) |
| C4 | 0.0168 (5) | 0.0118 (5) | 0.0193 (5) | −0.0004 (6) | 0.0079 (4) | −0.0007 (7) |
| C5 | 0.0145 (7) | 0.0144 (7) | 0.0200 (9) | 0.0000 (5) | 0.0076 (7) | 0.0009 (6) |
| C6 | 0.0140 (7) | 0.0137 (6) | 0.0188 (8) | −0.0005 (5) | 0.0068 (6) | 0.0000 (6) |
| C7 | 0.0193 (6) | 0.0118 (5) | 0.0276 (6) | 0.0029 (7) | 0.0092 (5) | −0.0007 (8) |
Geometric parameters (Å, º) top
| O1—C7 | 1.222 (2) | C2—H2 | 0.9500 |
| O2—B1 | 1.370 (2) | C3—C4 | 1.398 (3) |
| O2—H2O | 0.87 (3) | C3—H3 | 0.9500 |
| O3—B1 | 1.363 (2) | C4—C5 | 1.401 (2) |
| O3—H3O | 0.83 (3) | C4—C7 | 1.4740 (17) |
| B1—C1 | 1.5724 (17) | C5—C6 | 1.392 (2) |
| C1—C2 | 1.403 (2) | C5—H5 | 0.9500 |
| C1—C6 | 1.407 (2) | C6—H6 | 0.9500 |
| C2—C3 | 1.394 (2) | C7—H7 | 0.9500 |
| | | |
| B1—O2—H2O | 111.8 (17) | C4—C3—H3 | 120.1 |
| B1—O3—H3O | 117.3 (19) | C3—C4—C5 | 120.21 (11) |
| O3—B1—O2 | 117.70 (11) | C3—C4—C7 | 119.34 (18) |
| O3—B1—C1 | 124.09 (16) | C5—C4—C7 | 120.45 (18) |
| O2—B1—C1 | 118.21 (15) | C6—C5—C4 | 119.43 (15) |
| C2—C1—C6 | 118.39 (10) | C6—C5—H5 | 120.3 |
| C2—C1—B1 | 121.06 (15) | C4—C5—H5 | 120.3 |
| C6—C1—B1 | 120.55 (16) | C5—C6—C1 | 121.24 (15) |
| C3—C2—C1 | 120.91 (14) | C5—C6—H6 | 119.4 |
| C3—C2—H2 | 119.5 | C1—C6—H6 | 119.4 |
| C1—C2—H2 | 119.5 | O1—C7—C4 | 123.21 (19) |
| C2—C3—C4 | 119.82 (15) | O1—C7—H7 | 118.4 |
| C2—C3—H3 | 120.1 | C4—C7—H7 | 118.4 |
| | | |
| O3—B1—C1—C2 | −20.6 (3) | C2—C3—C4—C7 | 180.00 (18) |
| O2—B1—C1—C2 | 159.8 (2) | C3—C4—C5—C6 | −0.2 (4) |
| O3—B1—C1—C6 | 159.0 (2) | C7—C4—C5—C6 | −179.48 (18) |
| O2—B1—C1—C6 | −20.6 (3) | C4—C5—C6—C1 | −0.6 (3) |
| C6—C1—C2—C3 | −0.3 (3) | C2—C1—C6—C5 | 0.8 (3) |
| B1—C1—C2—C3 | 179.28 (17) | B1—C1—C6—C5 | −178.76 (16) |
| C1—C2—C3—C4 | −0.4 (3) | C3—C4—C7—O1 | 178.8 (2) |
| C2—C3—C4—C5 | 0.7 (4) | C5—C4—C7—O1 | −1.8 (4) |
Hydrogen-bond geometry (Å, º) top
| D—H···A | D—H | H···A | D···A | D—H···A |
| O2—H2O···O1i | 0.87 (3) | 1.86 (3) | 2.7209 (16) | 171 (3) |
| O3—H3O···O2ii | 0.83 (3) | 2.02 (3) | 2.8321 (13) | 165 (3) |
| Symmetry codes: (i) x, y+1, z; (ii) x+1/2, −y+3/2, z+1/2. |
Experimental details
| Crystal data |
| Chemical formula | C7H7BO3 |
| Mr | 149.94 |
| Crystal system, space group | Monoclinic, Cc |
| Temperature (K) | 120 |
| a, b, c (Å) | 11.1238 (3), 9.8718 (3), 7.1988 (2) |
| β (°) | 119.071 (2) |
| V (Å3) | 690.92 (3) |
| Z | 4 |
| Radiation type | Mo Kα |
| µ (mm−1) | 0.11 |
| Crystal size (mm) | 0.37 × 0.25 × 0.22 |
| |
| Data collection |
| Diffractometer | Nonius KappaCCD (with Oxford Cryostream)
diffractometer |
| Absorption correction | – |
No. of measured, independent and
observed [I > 2σ(I)] reflections | 4518, 1192, 1110 |
| Rint | 0.021 |
| (sin θ/λ)max (Å−1) | 0.746 |
| |
| Refinement |
| R[F2 > 2σ(F2)], wR(F2), S | 0.038, 0.104, 1.07 |
| No. of reflections | 1192 |
| No. of parameters | 106 |
| No. of restraints | 2 |
| H-atom treatment | H atoms treated by a mixture of independent and constrained refinement |
| Δρmax, Δρmin (e Å−3) | 0.44, −0.22 |
Selected geometric parameters (Å, º) top| O1—C7 | 1.222 (2) | O3—B1 | 1.363 (2) |
| O2—B1 | 1.370 (2) | B1—C1 | 1.5724 (17) |
| | | |
| O3—B1—O2 | 117.70 (11) | O2—B1—C1 | 118.21 (15) |
| O3—B1—C1 | 124.09 (16) | O1—C7—C4 | 123.21 (19) |
| | | |
| O3—B1—C1—C2 | −20.6 (3) | C5—C4—C7—O1 | −1.8 (4) |
Hydrogen-bond geometry (Å, º) top
| D—H···A | D—H | H···A | D···A | D—H···A |
| O2—H2O···O1i | 0.87 (3) | 1.86 (3) | 2.7209 (16) | 171 (3) |
| O3—H3O···O2ii | 0.83 (3) | 2.02 (3) | 2.8321 (13) | 165 (3) |
| Symmetry codes: (i) x, y+1, z; (ii) x+1/2, −y+3/2, z+1/2. |

 journal menu
journal menu








![[Figure 1]](http://journals.iucr.org/c/issues/2001/12/00/da1214/da1214fig1thm.gif)
![[Figure 2]](http://journals.iucr.org/c/issues/2001/12/00/da1214/da1214fig2thm.gif)




During the course of studying the structure and the mechanism of formation of colored products in resorcinarene solutions (Davis et al., 1999; Lewis et al., 2000), the model compound, (I), was investigated. Thermolysis of (I) led to the formation of the title compound, (II), and its structure was determined to ascertain its identity. \sch
The published crystal structure of (II) (Feulner et al., 1990) is in space group P1 with Z' = 1 at 293 K, and has some unsettling features. In their model, the CHO group has a twofold disorder which superimposes its C—H and C═O bonds. The boronic acid H-atom positions are not sensible for the expected hydrogen bonding, and form impossibly short intermolecular H···H contacts. Their model fits the data poorly (R = 0.097 and wR = 0.181), despite the fact that this compound forms high quality crystals. Furthermore, the triclinic cell can be transformed (011,011,100) to a C-centered cell with a monoclinic metric. Feulner et al. recognized this transformation, and attempted a structure solution in C2/c with Z' = 1/2. Their reported C-centered cell has dimensions a = 11.177 (5), b = 9.891 (4) and c = 7.339 (4) Å, and β = 118.37 (3)° (Note: transformation of their triclinic cell yields β = 119.11°, which more closely matches our value). Their model, deposited as the `monoclinic form' (refcode VEXFUZ01) in the Cambridge Structural Database (Allen & Kennard, 1993) has the molecule on a twofold axis, which requires a similar disorder in the formyl group. This model produced worse R values (R = 0.145 and wR = 0.151). Despite intensity statistics suggesting a centrosymmetric structure, Feulner et al. also attempted structure solution in space group Cc, but were unsuccessful for reasons which are unclear.
Our structure of (II), with Z' = 1 in space group Cc (Fig. 1), exhibits none of these troubling features. The formyl group is ordered, and the H atoms of the B(OH)2 group are ordered and in sensible positions for intermolecular hydrogen bonds (Table 2). The packing (Fig. 2) exhibits a pseudocenter near (1/2,1/2,1/2) and a pseudo-twofold axis near (1/2,y,3/4), running along the long axis of the molecule. The two molecules near the center of the cell are related by the c glide, and are approximately related by the pseudocenter. Treating the center as exact rather than the glide leads to the P1 model, while treating both the center and the glide as exact leads to the C2/c model. The cause of the disordered formyl group and boronic acid H atoms in the P1 model can be seen in Fig. 2 by examination of the relative orientations of the two glide-related molecules about (1/2,1/2,1/2). The formyl O and boronic acid H atoms do not conform to the inversion, while the remainder of the molecule nearly does. The pseudosymmetry does not lead to exceptionally high correlations, with the largest being 0.63, between displacement parameters of atoms related by the approximate twofold axis.
We have ruled out the possibility that a phase change on cooling causes the difference between the Cc structure which we observe at 120 K and that reported by Feulner et al. at room temperature. Using the same crystal, we collected intensity data at 296 K and obtained the same Cc structure, with cell dimensions a = 11.1932 (4), b = 9.8820 (5) and c = 7.3373 (3) Å, β = 119.336 (3)° and R = 0.043. Using this data set, we were also able to reproduce the results of Feulner et al., refining their P1 model to R = 0.099.
The structure of the molecule itself is unremarkable. The formyl group is essentially coplanar with the phenyl ring, while the B(OH)2 group is rotated by 20.6 (3)° out of the phenyl plane. One hydroxyl H atom is syn to the phenyl group, while the other is anti, as is typical for phenylboronic acids (Bradley et al., 1996; Gainsford et al., 1995; Pilkington et al., 1995; Shull et al., 2000; Soundararajan et al., 1993), including unsubstituted phenylboronic acid (Rettig & Trotter, 1977) and the ortho isomer of the title compound (Scouten et al., 1994).
Baur & Kassner (1992) and Marsh (1997) have warned of the perils of space group Cc. In the present structure, more perilous is the imposition of centrosymmetry on the basis of centric intensity statistics. From our data, a chemically correct structure, albeit unnecessarily low symmetry, may be easily obtained in space group P1 Not P1?. However, no chemically correct model can be obtained in any centrosymmetric space group.