Buy article online - an online subscription or single-article purchase is required to access this article.

Molecular crystals exhibiting polar symmetry are important paradigms for developing new electrooptical materials. Though accessing bulk polarity still presents a significant challenge, in some cases it may be rationalized as being associated with the specific molecular shapes and symmetries and subtle features of supramolecular interactions. In the crystal structure of 3,5,7-trinitro-1-azaadamantane, C
9H
12N
4O
6, the polar symmetry of the molecular arrangement is a result of complementary prerequisites, namely the
C3v symmetry of the molecules is suited to the generation of polar stacks and the inherent asymmetry of the principal supramolecular bonding, as is provided by NO
2(lone pair)

NO
2(π-hole) interactions. These bonds arrange the molecules into a trigonal network. In spite of the apparent simplicity, the structure comprises three unique molecules (
Z′ =
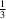
+
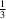
+
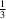
), two of which are donors and acceptors of three N

O interactions and the third being primarily important for weak C—H

O hydrogen bonding. These distinct structural roles agree with the results of Hirshfeld surface analysis. A set of weak C—H

O and C—H

N hydrogen bonds yields three kinds of stacks. The orientation of the stacks is identical and therefore the polarity of each molecule contributes additively to the net dipole moment of the crystal. This suggests a special potential of asymmetric NO
2(lone pair)

NO
2(π-hole) interactions for the supramolecular synthesis of acentric materials.
Supporting information
CCDC reference: 2004782
Data collection: IPDS (Stoe & Cie, 2000); cell refinement: IPDS (Stoe & Cie, 2000); data reduction: IPDS (Stoe & Cie, 2000); program(s) used to solve structure: SHELXS97 (Sheldrick, 2008); program(s) used to refine structure: SHELXL2018 (Sheldrick, 2015); molecular graphics: DIAMOND (Brandenburg, 1999); software used to prepare material for publication: WinGX (Farrugia, 1999).
2,5,7-Trinitro-1-azatricyclo[3.3.1.13.7]decane
top
Crystal data top
| C9H12N4O6 | Dx = 1.622 Mg m−3 |
| Mr = 272.23 | Mo Kα radiation, λ = 0.71073 Å |
| Trigonal, P3 | Cell parameters from 5172 reflections |
| a = 12.7917 (14) Å | θ = 3.2–29.2° |
| c = 5.8996 (6) Å | µ = 0.14 mm−1 |
| V = 836.0 (2) Å3 | T = 200 K |
| Z = 3 | Prism, colorless |
| F(000) = 426 | 0.22 × 0.20 × 0.17 mm |
Data collection top
Stoe Image plate diffraction system IPDS-2T
diffractometer | 2451 reflections with I > 2σ(I) |
| Radiation source: fine-focus sealed tube | Rint = 0.038 |
| φ oscillation scans | θmax = 29.2°, θmin = 3.2° |
Absorption correction: numerical
[X-RED (Stoe & Cie, 2001) and X-SHAPE (Stoe & Cie, 1999)] | h = −17→16 |
| Tmin = 0.654, Tmax = 0.792 | k = −17→15 |
| 5172 measured reflections | l = −8→8 |
| 2961 independent reflections | |
Refinement top
| Refinement on F2 | Primary atom site location: structure-invariant direct methods |
| Least-squares matrix: full | Secondary atom site location: difference Fourier map |
| R[F2 > 2σ(F2)] = 0.032 | Hydrogen site location: difference Fourier map |
| wR(F2) = 0.063 | All H-atom parameters refined |
| S = 0.92 | w = 1/[σ2(Fo2) + (0.033P)2]
where P = (Fo2 + 2Fc2)/3 |
| 2961 reflections | (Δ/σ)max < 0.001 |
| 221 parameters | Δρmax = 0.14 e Å−3 |
| 1 restraint | Δρmin = −0.19 e Å−3 |
Special details top
Geometry. All esds (except the esd in the dihedral angle between two l.s. planes)
are estimated using the full covariance matrix. The cell esds are taken
into account individually in the estimation of esds in distances, angles
and torsion angles; correlations between esds in cell parameters are only
used when they are defined by crystal symmetry. An approximate (isotropic)
treatment of cell esds is used for estimating esds involving l.s. planes. |
Refinement. Refined as a 2-component twin. |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2) top| | x | y | z | Uiso*/Ueq | |
| O1A | 0.2573 (3) | 0.2891 (2) | −0.2900 (6) | 0.0475 (7) | |
| O2A | 0.3275 (2) | 0.1737 (3) | −0.1919 (5) | 0.0413 (6) | |
| N1A | 0.000000 | 0.000000 | 0.1998 (7) | 0.0183 (9) | |
| N2A | 0.2452 (3) | 0.1945 (3) | −0.2164 (4) | 0.0267 (6) | |
| C1A | 0.1201 (3) | 0.0952 (3) | 0.1220 (5) | 0.0205 (6) | |
| C2A | 0.1207 (3) | 0.0967 (3) | −0.1406 (5) | 0.0196 (6) | |
| C3A | 0.0253 (3) | 0.1249 (3) | −0.2281 (5) | 0.0203 (6) | |
| H1A | 0.182 (3) | 0.077 (3) | 0.183 (5) | 0.019 (8)* | |
| H2A | 0.131 (3) | 0.167 (4) | 0.173 (6) | 0.017 (8)* | |
| H3A | 0.039 (3) | 0.197 (4) | −0.180 (5) | 0.011 (8)* | |
| H4A | 0.024 (3) | 0.118 (3) | −0.396 (6) | 0.018 (8)* | |
| O1B | 0.3760 (2) | 0.3259 (3) | 0.2319 (5) | 0.0362 (6) | |
| O2B | 0.5076 (3) | 0.4797 (3) | 0.0412 (6) | 0.0488 (8) | |
| N1B | 0.666667 | 0.333333 | 0.5825 (7) | 0.0224 (10) | |
| N2B | 0.4789 (3) | 0.3926 (3) | 0.1637 (4) | 0.0259 (6) | |
| C1B | 0.5760 (3) | 0.3643 (3) | 0.5048 (5) | 0.0241 (6) | |
| C2B | 0.5754 (3) | 0.3651 (3) | 0.2425 (5) | 0.0178 (6) | |
| C3B | 0.5402 (3) | 0.2387 (3) | 0.1525 (5) | 0.0183 (6) | |
| H1B | 0.599 (3) | 0.443 (4) | 0.554 (5) | 0.016 (8)* | |
| H2B | 0.506 (3) | 0.312 (3) | 0.567 (5) | 0.008 (7)* | |
| H3B | 0.466 (3) | 0.182 (3) | 0.207 (5) | 0.012 (8)* | |
| H4B | 0.549 (4) | 0.243 (3) | −0.006 (6) | 0.024 (9)* | |
| O1C | 0.1491 (3) | 0.3784 (3) | 0.2511 (5) | 0.0462 (7) | |
| O2C | 0.0360 (3) | 0.3839 (3) | 0.5158 (6) | 0.0521 (9) | |
| N1C | 0.333333 | 0.666667 | 0.8362 (8) | 0.0264 (11) | |
| N2C | 0.1322 (3) | 0.4261 (2) | 0.4153 (5) | 0.0264 (6) | |
| C1C | 0.2356 (3) | 0.5486 (3) | 0.7592 (5) | 0.0248 (7) | |
| C2C | 0.2346 (3) | 0.5470 (3) | 0.4983 (5) | 0.0201 (6) | |
| C3C | 0.3551 (3) | 0.5649 (3) | 0.4084 (5) | 0.0205 (6) | |
| H1C | 0.160 (4) | 0.543 (3) | 0.807 (6) | 0.025 (9)* | |
| H2C | 0.245 (4) | 0.484 (4) | 0.813 (6) | 0.024 (9)* | |
| H3C | 0.347 (3) | 0.562 (3) | 0.242 (5) | 0.010 (8)* | |
| H4C | 0.370 (3) | 0.504 (3) | 0.463 (5) | 0.014 (7)* | |
Atomic displacement parameters (Å2) top| | U11 | U22 | U33 | U12 | U13 | U23 |
| O1A | 0.0353 (15) | 0.0306 (15) | 0.0659 (16) | 0.0084 (12) | 0.0138 (14) | 0.0198 (14) |
| O2A | 0.0191 (13) | 0.0453 (16) | 0.0532 (15) | 0.0113 (12) | 0.0059 (11) | 0.0085 (13) |
| N1A | 0.0182 (14) | 0.0182 (14) | 0.019 (2) | 0.0091 (7) | 0.000 | 0.000 |
| N2A | 0.0199 (14) | 0.0239 (14) | 0.0246 (13) | 0.0022 (12) | 0.0032 (10) | 0.0011 (11) |
| C1A | 0.0189 (16) | 0.0209 (16) | 0.0188 (13) | 0.0078 (13) | −0.0014 (12) | −0.0020 (12) |
| C2A | 0.0191 (15) | 0.0211 (15) | 0.0188 (12) | 0.0102 (13) | −0.0002 (12) | 0.0010 (11) |
| C3A | 0.0226 (15) | 0.0201 (16) | 0.0194 (15) | 0.0116 (13) | 0.0018 (11) | 0.0028 (12) |
| O1B | 0.0184 (12) | 0.0408 (15) | 0.0510 (15) | 0.0161 (12) | 0.0015 (10) | −0.0020 (12) |
| O2B | 0.0373 (17) | 0.0530 (19) | 0.0662 (17) | 0.0300 (15) | 0.0032 (13) | 0.0265 (15) |
| N1B | 0.0256 (16) | 0.0256 (16) | 0.016 (2) | 0.0128 (8) | 0.000 | 0.000 |
| N2B | 0.0243 (15) | 0.0290 (16) | 0.0296 (14) | 0.0173 (12) | −0.0024 (11) | −0.0039 (12) |
| C1B | 0.0257 (18) | 0.0283 (17) | 0.0197 (13) | 0.0146 (15) | 0.0058 (12) | 0.0023 (13) |
| C2B | 0.0150 (14) | 0.0193 (14) | 0.0208 (13) | 0.0099 (12) | −0.0022 (10) | −0.0019 (11) |
| C3B | 0.0164 (15) | 0.0180 (15) | 0.0197 (14) | 0.0079 (12) | −0.0014 (11) | −0.0011 (12) |
| O1C | 0.0374 (16) | 0.0329 (15) | 0.0592 (17) | 0.0107 (14) | −0.0005 (13) | −0.0216 (13) |
| O2C | 0.0251 (15) | 0.0407 (19) | 0.067 (2) | −0.0013 (14) | 0.0101 (13) | −0.0094 (15) |
| N1C | 0.0315 (17) | 0.0315 (17) | 0.016 (2) | 0.0157 (9) | 0.000 | 0.000 |
| N2C | 0.0235 (15) | 0.0186 (14) | 0.0358 (15) | 0.0094 (13) | −0.0017 (12) | 0.0001 (11) |
| C1C | 0.0251 (18) | 0.0264 (18) | 0.0221 (15) | 0.0123 (14) | 0.0047 (13) | 0.0051 (12) |
| C2C | 0.0171 (16) | 0.0156 (15) | 0.0236 (15) | 0.0052 (12) | 0.0005 (12) | −0.0008 (11) |
| C3C | 0.0201 (15) | 0.0191 (15) | 0.0230 (14) | 0.0103 (13) | 0.0018 (12) | 0.0012 (11) |
Geometric parameters (Å, º) top
| O1A—N2A | 1.220 (4) | C1B—H1B | 0.94 (4) |
| O2A—N2A | 1.217 (4) | C1B—H2B | 0.89 (3) |
| N1A—C1Ai | 1.478 (3) | C2B—C3Biv | 1.523 (4) |
| N1A—C1Aii | 1.478 (3) | C2B—C3B | 1.540 (4) |
| N1A—C1A | 1.478 (3) | C3B—H3B | 0.92 (3) |
| N2A—C2A | 1.519 (4) | C3B—H4B | 0.94 (4) |
| C1A—C2A | 1.549 (4) | O1C—N2C | 1.220 (4) |
| C1A—H1A | 1.00 (4) | O2C—N2C | 1.222 (4) |
| C1A—H2A | 0.91 (4) | N1C—C1C | 1.470 (4) |
| C2A—C3A | 1.525 (5) | N1C—C1Cv | 1.470 (4) |
| C2A—C3Ai | 1.534 (4) | N1C—C1Cvi | 1.470 (4) |
| C3A—H3A | 0.89 (4) | N2C—C2C | 1.524 (4) |
| C3A—H4A | 0.99 (3) | C1C—C2C | 1.539 (4) |
| O1B—N2B | 1.225 (4) | C1C—H1C | 0.97 (4) |
| O2B—N2B | 1.219 (4) | C1C—H2C | 0.95 (4) |
| N1B—C1Biii | 1.473 (4) | C2C—C3Cv | 1.532 (4) |
| N1B—C1Biv | 1.473 (4) | C2C—C3C | 1.535 (5) |
| N1B—C1B | 1.473 (4) | C3C—H3C | 0.99 (3) |
| N2B—C2B | 1.517 (4) | C3C—H4C | 0.94 (4) |
| C1B—C2B | 1.548 (4) | | |
| | | |
| C1Ai—N1A—C1Aii | 110.82 (19) | N2B—C2B—C3Biv | 110.2 (2) |
| C1Ai—N1A—C1A | 110.82 (19) | N2B—C2B—C3B | 107.1 (2) |
| C1Aii—N1A—C1A | 110.82 (19) | C3Biv—C2B—C3B | 111.0 (3) |
| O2A—N2A—O1A | 124.4 (3) | N2B—C2B—C1B | 108.4 (2) |
| O2A—N2A—C2A | 116.7 (3) | C3Biv—C2B—C1B | 110.3 (2) |
| O1A—N2A—C2A | 118.9 (3) | C3B—C2B—C1B | 109.7 (3) |
| N1A—C1A—C2A | 108.5 (3) | C2Biii—C3B—C2B | 106.2 (3) |
| N1A—C1A—H1A | 108 (2) | C2Biii—C3B—H3B | 109 (2) |
| C2A—C1A—H1A | 111.4 (19) | C2B—C3B—H3B | 110 (2) |
| N1A—C1A—H2A | 107 (2) | C2Biii—C3B—H4B | 106 (2) |
| C2A—C1A—H2A | 109 (2) | C2B—C3B—H4B | 108 (2) |
| H1A—C1A—H2A | 112 (3) | H3B—C3B—H4B | 116 (3) |
| N2A—C2A—C3A | 109.8 (3) | C1C—N1C—C1Cv | 110.9 (2) |
| N2A—C2A—C3Ai | 108.5 (3) | C1C—N1C—C1Cvi | 110.9 (2) |
| C3A—C2A—C3Ai | 111.8 (3) | C1Cv—N1C—C1Cvi | 110.9 (2) |
| N2A—C2A—C1A | 107.5 (2) | O1C—N2C—O2C | 123.7 (3) |
| C3A—C2A—C1A | 110.0 (3) | O1C—N2C—C2C | 119.1 (3) |
| C3Ai—C2A—C1A | 109.1 (3) | O2C—N2C—C2C | 117.2 (3) |
| C2A—C3A—C2Aii | 106.6 (3) | N1C—C1C—C2C | 108.7 (3) |
| C2A—C3A—H3A | 112 (2) | N1C—C1C—H1C | 107 (2) |
| C2Aii—C3A—H3A | 111 (2) | C2C—C1C—H1C | 107 (2) |
| C2A—C3A—H4A | 107 (2) | N1C—C1C—H2C | 112 (2) |
| C2Aii—C3A—H4A | 107.5 (19) | C2C—C1C—H2C | 109 (2) |
| H3A—C3A—H4A | 113 (3) | H1C—C1C—H2C | 113 (3) |
| C1Biii—N1B—C1Biv | 110.8 (2) | N2C—C2C—C3Cv | 107.1 (2) |
| C1Biii—N1B—C1B | 110.8 (2) | N2C—C2C—C3C | 109.2 (3) |
| C1Biv—N1B—C1B | 110.8 (2) | C3Cv—C2C—C3C | 111.1 (3) |
| O2B—N2B—O1B | 124.0 (3) | N2C—C2C—C1C | 109.4 (3) |
| O2B—N2B—C2B | 119.0 (3) | C3Cv—C2C—C1C | 109.8 (2) |
| O1B—N2B—C2B | 117.0 (3) | C3C—C2C—C1C | 110.0 (3) |
| N1B—C1B—C2B | 108.7 (3) | C2Cvi—C3C—C2C | 106.2 (3) |
| N1B—C1B—H1B | 109 (2) | C2Cvi—C3C—H3C | 113 (2) |
| C2B—C1B—H1B | 107.5 (19) | C2C—C3C—H3C | 104.9 (18) |
| N1B—C1B—H2B | 108 (2) | C2Cvi—C3C—H4C | 109 (2) |
| C2B—C1B—H2B | 114.3 (19) | C2C—C3C—H4C | 111 (2) |
| H1B—C1B—H2B | 109 (3) | H3C—C3C—H4C | 112 (3) |
| | | |
| C1Ai—N1A—C1A—C2A | 62.2 (3) | O1B—N2B—C2B—C1B | −56.3 (4) |
| C1Aii—N1A—C1A—C2A | −61.3 (3) | N1B—C1B—C2B—N2B | 178.0 (2) |
| O2A—N2A—C2A—C3A | −168.0 (3) | N1B—C1B—C2B—C3Biv | −61.2 (3) |
| O1A—N2A—C2A—C3A | 14.4 (4) | N1B—C1B—C2B—C3B | 61.4 (3) |
| O2A—N2A—C2A—C3Ai | −45.5 (4) | N2B—C2B—C3B—C2Biii | −177.4 (2) |
| O1A—N2A—C2A—C3Ai | 136.9 (3) | C3Biv—C2B—C3B—C2Biii | 62.2 (4) |
| O2A—N2A—C2A—C1A | 72.4 (4) | C1B—C2B—C3B—C2Biii | −60.0 (3) |
| O1A—N2A—C2A—C1A | −105.2 (4) | C1Cv—N1C—C1C—C2C | 62.0 (3) |
| N1A—C1A—C2A—N2A | −179.6 (2) | C1Cvi—N1C—C1C—C2C | −61.7 (3) |
| N1A—C1A—C2A—C3A | 60.9 (3) | O1C—N2C—C2C—C3Cv | 100.4 (3) |
| N1A—C1A—C2A—C3Ai | −62.1 (3) | O2C—N2C—C2C—C3Cv | −77.8 (4) |
| N2A—C2A—C3A—C2Aii | −178.8 (2) | O1C—N2C—C2C—C3C | −20.1 (4) |
| C3Ai—C2A—C3A—C2Aii | 60.7 (4) | O2C—N2C—C2C—C3C | 161.7 (3) |
| C1A—C2A—C3A—C2Aii | −60.7 (3) | O1C—N2C—C2C—C1C | −140.6 (3) |
| C1Biii—N1B—C1B—C2B | −61.9 (3) | O2C—N2C—C2C—C1C | 41.2 (4) |
| C1Biv—N1B—C1B—C2B | 61.4 (3) | N1C—C1C—C2C—N2C | −178.9 (2) |
| O2B—N2B—C2B—C3Biv | 1.6 (4) | N1C—C1C—C2C—C3Cv | −61.6 (3) |
| O1B—N2B—C2B—C3Biv | −177.1 (3) | N1C—C1C—C2C—C3C | 61.1 (3) |
| O2B—N2B—C2B—C3B | −119.2 (3) | N2C—C2C—C3C—C2Cvi | 180.0 (2) |
| O1B—N2B—C2B—C3B | 62.0 (3) | C3Cv—C2C—C3C—C2Cvi | 62.0 (4) |
| O2B—N2B—C2B—C1B | 122.4 (3) | C1C—C2C—C3C—C2Cvi | −59.9 (3) |
| Symmetry codes: (i) −x+y, −x, z; (ii) −y, x−y, z; (iii) −y+1, x−y, z; (iv) −x+y+1, −x+1, z; (v) −x+y, −x+1, z; (vi) −y+1, x−y+1, z. |
Hydrogen-bond geometry (Å, º) top
| D—H···A | D—H | H···A | D···A | D—H···A |
| C1A—H2A···O1C | 0.90 (4) | 2.64 (4) | 3.536 (5) | 170 (3) |
| C3A—H4A···N1Avii | 0.99 (3) | 2.76 (3) | 3.678 (5) | 154 (3) |
| C1B—H1B···O2Cvi | 0.94 (4) | 2.58 (4) | 3.455 (5) | 154 (3) |
| C1B—H2B···O2Aviii | 0.89 (3) | 2.51 (3) | 3.392 (4) | 170 (2) |
| C3B—H4B···N1Bvii | 0.94 (4) | 2.79 (4) | 3.664 (5) | 156 (3) |
| C1C—H1C···O2B | 0.98 (4) | 2.56 (4) | 3.496 (4) | 161 (3) |
| C1C—H2C···O1Aviii | 0.96 (4) | 2.64 (4) | 3.479 (5) | 147 (3) |
| C3C—H4C···O1Aviii | 0.94 (4) | 2.80 (3) | 3.574 (4) | 140 (3) |
| C3C—H4C···O1B | 0.94 (4) | 2.69 (3) | 3.365 (4) | 129 (2) |
| C3C—H3C···N1Cvii | 0.98 (3) | 2.79 (3) | 3.678 (5) | 150 (3) |
| Symmetry codes: (vi) −y+1, x−y+1, z; (vii) x, y, z−1; (viii) x, y, z+1. |
Calculated interaction energies (kJ mol-1). top| Typea | Path | R (Å) | Eele | Epol | Edis | Erep | Etot |
| Type 1 | A···B | 7.72 | -9.4 | -2.2 | -18.7 | 13.4 | -19.7 |
| Type 2 | A···Bvii | 8.23 | -9.3 | -1.8 | -10.9 | 11.9 | -13.3 |
| A···C | 8.27 | -8.1 | -1.6 | -9.1 | 6.1 | -13.9 |
| B···Cvii | 8.60 | -7.0 | -1.1 | -7.1 | 5.3 | -11.1 |
| Type 3 | A···Cvii | 7.70 | -8.8 | -2.2 | -16.5 | 9.0 | -19.7 |
| B···C | 7.53 | -8.4 | -2.9 | -21.3 | 14.4 | -20.7 |
| Type 4 | A···Avii | 5.90 | -5.7 | -5.3 | -32.6 | 18.8 | -26.7 |
| B···Bvii | 5.90 | -9.0 | -5.3 | -34.6 | 20.8 | -30.8 |
| C···Cvii | 5.90 | -11.6 | -4.0 | -35.8 | 22.0 | -32.8 |
| Interaction energies were calculated employing the CE-B3LYP/6-31G(d,p)
functional/basis set combination. The scale factors used to determine
Etot: kele = 1.057, kpol = 0.740, kdis =
0.871 and krep = 0.618 (Mackenzie et al., 2017).
Note: (a) for details of the interaction modes, See Fig. 6;
R is the distance between the centroids of interacting molecules.
[Symmetry code: (vii) x, y, z-1.] |

 Subscribe to Acta Crystallographica Section C: Structural Chemistry
Subscribe to Acta Crystallographica Section C: Structural Chemistry
The full text of this article is available to subscribers to the journal.
If you have already registered and are using a computer listed in your registration details, please email
support@iucr.org for assistance.
 NO2(π-hole) interactions. These bonds arrange the molecules into a trigonal network. In spite of the apparent simplicity, the structure comprises three unique molecules (Z′ =
NO2(π-hole) interactions. These bonds arrange the molecules into a trigonal network. In spite of the apparent simplicity, the structure comprises three unique molecules (Z′ = 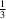 +
+ 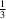 +
+ 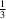 ), two of which are donors and acceptors of three N
), two of which are donors and acceptors of three N O interactions and the third being primarily important for weak C—H
O interactions and the third being primarily important for weak C—H O hydrogen bonding. These distinct structural roles agree with the results of Hirshfeld surface analysis. A set of weak C—H
O hydrogen bonding. These distinct structural roles agree with the results of Hirshfeld surface analysis. A set of weak C—H O and C—H
O and C—H N hydrogen bonds yields three kinds of stacks. The orientation of the stacks is identical and therefore the polarity of each molecule contributes additively to the net dipole moment of the crystal. This suggests a special potential of asymmetric NO2(lone pair)
N hydrogen bonds yields three kinds of stacks. The orientation of the stacks is identical and therefore the polarity of each molecule contributes additively to the net dipole moment of the crystal. This suggests a special potential of asymmetric NO2(lone pair) NO2(π-hole) interactions for the supramolecular synthesis of acentric materials.
NO2(π-hole) interactions for the supramolecular synthesis of acentric materials. NO2(π-hole) interactions. These bonds arrange the molecules into a trigonal network. In spite of the apparent simplicity, the structure comprises three unique molecules (Z′ =
NO2(π-hole) interactions. These bonds arrange the molecules into a trigonal network. In spite of the apparent simplicity, the structure comprises three unique molecules (Z′ =  O interactions and the third being primarily important for weak C—H
O interactions and the third being primarily important for weak C—H O hydrogen bonding. These distinct structural roles agree with the results of Hirshfeld surface analysis. A set of weak C—H
O hydrogen bonding. These distinct structural roles agree with the results of Hirshfeld surface analysis. A set of weak C—H O and C—H
O and C—H N hydrogen bonds yields three kinds of stacks. The orientation of the stacks is identical and therefore the polarity of each molecule contributes additively to the net dipole moment of the crystal. This suggests a special potential of asymmetric NO2(lone pair)
N hydrogen bonds yields three kinds of stacks. The orientation of the stacks is identical and therefore the polarity of each molecule contributes additively to the net dipole moment of the crystal. This suggests a special potential of asymmetric NO2(lone pair) NO2(π-hole) interactions for the supramolecular synthesis of acentric materials.
NO2(π-hole) interactions for the supramolecular synthesis of acentric materials.
 journal menu
journal menu









 Subscribe to Acta Crystallographica Section C: Structural Chemistry
Subscribe to Acta Crystallographica Section C: Structural Chemistry


