Buy article online - an online subscription or single-article purchase is required to access this article.
Bursts of emissions of low-energy electrons, including interatomic Coulomb decay electrons and Auger electrons (0-1000 eV), as well as X-ray fluorescence produced by irradiation of large-Z element nanoparticles by either X-ray photons or high-energy ion beams, is referred to as the nanoradiator effect. In therapeutic applications, this effect can damage pathological tissues that selectively take up the nanoparticles. Herein, a new nanoradiator dosimetry method is presented that uses probes for reactive oxygen species (ROS) incorporated into three-dimensional gels, on which macrophages containing iron oxide nanoparticles (IONs) are attached. This method, together with site-specific irradiation of the intracellular nanoparticles from a microbeam of polychromatic synchrotron X-rays (5-14 keV), measures the range and distribution of OH radicals produced by X-ray emission or superoxide anions (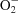 ) produced by low-energy electrons. The measurements are based on confocal laser scanning of the fluorescence of the hydroxyl radical probe 2-[6-(4'-amino)phenoxy-3H-xanthen-3-on-9-yl] benzoic acid (APF) or the superoxide probe hydroethidine-dihydroethidium (DHE) that was oxidized by each ROS, enabling tracking of the radiation dose emitted by the nanoradiator. In the range 70 µm below the irradiated cell,
) produced by low-energy electrons. The measurements are based on confocal laser scanning of the fluorescence of the hydroxyl radical probe 2-[6-(4'-amino)phenoxy-3H-xanthen-3-on-9-yl] benzoic acid (APF) or the superoxide probe hydroethidine-dihydroethidium (DHE) that was oxidized by each ROS, enabling tracking of the radiation dose emitted by the nanoradiator. In the range 70 µm below the irradiated cell, 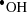 radicals derived mostly from either incident X-ray or X-ray fluorescence of ION nanoradiators are distributed along the line of depth direction in ROS gel. In contrast,
radicals derived mostly from either incident X-ray or X-ray fluorescence of ION nanoradiators are distributed along the line of depth direction in ROS gel. In contrast, 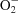 derived from secondary electron or low-energy electron emission by ION nanoradiators are scattered over the ROS gel. ROS fluorescence due to the ION nanoradiators was observed continuously to a depth of 1.5 mm for both oxidized APF and oxidized DHE with relatively large intensity compared with the fluorescence caused by the ROS produced solely by incident primary X-rays, which was limited to a depth of 600 µm, suggesting dose enhancement as well as more penetration by nanoradiators. In conclusion, the combined use of a synchrotron X-ray microbeam-irradiated three-dimensional ROS gel and confocal laser scanning fluorescence microscopy provides a simple dosimetry method for track analysis of X-ray photoelectric nanoradiator radiation, suggesting extensive cellular damage with dose-enhancement beyond a single cell containing IONs.
derived from secondary electron or low-energy electron emission by ION nanoradiators are scattered over the ROS gel. ROS fluorescence due to the ION nanoradiators was observed continuously to a depth of 1.5 mm for both oxidized APF and oxidized DHE with relatively large intensity compared with the fluorescence caused by the ROS produced solely by incident primary X-rays, which was limited to a depth of 600 µm, suggesting dose enhancement as well as more penetration by nanoradiators. In conclusion, the combined use of a synchrotron X-ray microbeam-irradiated three-dimensional ROS gel and confocal laser scanning fluorescence microscopy provides a simple dosimetry method for track analysis of X-ray photoelectric nanoradiator radiation, suggesting extensive cellular damage with dose-enhancement beyond a single cell containing IONs.

 journal menu
journal menu









 Subscribe to Journal of Synchrotron Radiation
Subscribe to Journal of Synchrotron Radiation


