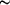 3.2 Å) Cu
3.2 Å) Cu Cu contacts within these compounds has been debated, being either described as weakly attractive (bonding) `cuprophilic' interactions, or simply as short metal-metal distances constrained by ligand geometry or largely ionic in nature. The title three-dimensional Cu+-containing coordination polymer, [Cu3(C7H7N2O2)Cl2]n, was formed from the in situ reduction of CuCl2 in the presence of 3,5-diaminobenzoic acid and KOH under hydrothermal conditions. Its complex crystal structure contains ten distinct CuI atoms, two of which lie on crystallographic inversion centres. The copper coordination geometries include near-linear CuOCl and CuN2, T-shaped CuOCl2 and distorted tetrahedral CuOCl3 groups. Each CuI atom is also associated with two adjacent metal atoms, with Cu
Cu contacts within these compounds has been debated, being either described as weakly attractive (bonding) `cuprophilic' interactions, or simply as short metal-metal distances constrained by ligand geometry or largely ionic in nature. The title three-dimensional Cu+-containing coordination polymer, [Cu3(C7H7N2O2)Cl2]n, was formed from the in situ reduction of CuCl2 in the presence of 3,5-diaminobenzoic acid and KOH under hydrothermal conditions. Its complex crystal structure contains ten distinct CuI atoms, two of which lie on crystallographic inversion centres. The copper coordination geometries include near-linear CuOCl and CuN2, T-shaped CuOCl2 and distorted tetrahedral CuOCl3 groups. Each CuI atom is also associated with two adjacent metal atoms, with Cu Cu distances varying from 2.7350 (14) to 3.2142 (13) Å; if all these are regarded as `cuprophilic' interactions, then infinite [
Cu distances varying from 2.7350 (14) to 3.2142 (13) Å; if all these are regarded as `cuprophilic' interactions, then infinite [ Cl hydrogen bonds.
Cl hydrogen bonds.Supporting information
Crystallographic Information File (CIF) https://doi.org/10.1107/S205322961502330X/sk3607sup1.cif | |
Structure factor file (CIF format) https://doi.org/10.1107/S205322961502330X/sk3607Isup2.hkl |
CCDC reference: 990165
Compounds containing copper(I) (electronic configuration [Ar]3d10) are of interest for their role in biological processes (Tsukihara et al., 1995) and the presence of short (< ~3.2 Å) Cu···Cu contacts in their crystal structures (Arkhireeva et al., 1990; Zheng et al., 2005; Sundararaman et al., 2005; Doshi et al., 2012, Cotton et al., 1998; Carvajal et al., 2004). The nature of these contacts has been debated, with some authors (Zheng et al., 2005; Sundararaman et al., 2005; Doshi et al., 2012) describing them as weakly attractive (bonding) `cuprophilic' interactions akin to the well-established argentophilic Ag···Ag (Barreiro et al., 2013) and aurophilic Au···Au (Schmidbaur & Schier, 2012) weak bonds in silver(I) and gold(I) compounds, respectively. However, other workers have questioned whether these Cu···Cu contacts represent bonds at all and suggested that the short metal–metal distances observed are either constrained by the ligand geometry (Cotton et al., 1998) or are largely ionic in nature (Carvajal et al., 2004).
Here, we describe the hydrothermal synthesis and crystal structure of the three-dimensional coordination polymer [Cu3(C7H7N2O2)Cl2]n, (1), which arose from the unexpected reduction of the CuCl2 starting material to Cu+ under basic conditions; C7H7N2O2- is the 3,5-diaminobenzoate anion.
A mixture of CuCl2 (134 mg, 1.0 mmol) and 3,5-diaminobenzoic acid (304 mg, 2.0 mmol) was added to 1 M KOH (2.0 ml) with stirring to yield a pale-blue solution. This mixture was heated to 423 K in a 23 ml Teflon-lined autoclave for 15 h and then cooled to room temperature over a period of several hours. Colourless blocks of (1) were recovered from the reaction by filtration and rinsing with water and acetone.
Crystal data, data collection and structure refinement details are summarized in Table 1. The crystal quality was only fair, which may correlate with the rather high residuals and the larger than expected difference-map features (Δρ min. max. = -2.7 and 3.4 e Å-3, respectively). The H atoms were placed geometrically (C—H = 0.95 Å and N—H = 0.92 Å) and refined as riding atoms, with the constraint Uiso(H) = Ueq(carrier). This structure has a strong subcell (C2/c, a = 13.077, b = 10.894, c = 7.209 Å, β = 94.64° and V = 1023.6 Å3), but refinements in this space group, in which the asymmetric unit contains two CuI atoms (one with symmetry 1), two Cl atoms and half a ligand molecule, led to much higher residuals [R(F) ~0.20], large difference Fourier peaks (~±6 e Å-3) in the vicinity of the CuI atoms and many systematic absence violations and was therefore rejected. The structural models were analysed and verified with PLATON (Spek, 2009).
The lack of colour of the crystals of (1) is consistent with the 3d10 electron configuration of the Cu+ ion (Doshi et al., 2012; Kappenstein & Hugel, 1978). The precise nature of the redox reaction that generated the cuprous ions is unknown, but similar reductions of Cu2+ → Cu+ in the presence of a nitrogen-containing ligand under hydrothermal conditions have been reported by other workers (Xin et al., 2013) and indeed this type of reduction has been described as a `recommended route' (Peng et al., 2010) to cuprous–halide compounds from Cu2+-containing starting materials. The asymmetric unit of (1), which contains no fewer than ten crystallographically independent CuI atoms (eight of which lie on general positions and two on crystallographic inversion centres), as well as three benzoate ligands and six chloride ions, is shown in Fig. 1.
The copper coordination geometries in (1) can be split into several distinct types. Atoms Cu1–Cu6 are bonded to various combinations of benzoate O atoms and chloride ions (Table 1). In this complex structure, all these CuI atoms are surrounded by a number of O and Cl atoms at distances around or slightly shorter that the Bondi (1964) van der Waals radii sum of Cu···O = 2.92 Å and Cu···Cl = 3.15 Å, but by using the Brown criterion of 0.04 of a bond-valence unit to indicate a significant chemical bond to a univalent cation (Brown, 2002), the following conclusions can be drawn: Cu1 and Cu2 are bonded to a benzoate O atom and two chloride ions in a distorted T-shape, whereas Cu3 and Cu5 are bonded to one O atom and three chloride ions in very distorted tetrahedral arrangements (Fig. 2). The Cu4 and Cu6 coordination geometries are ambiguous; they have two well-defined short bonds to one O and one chloride ion in an approximately linear arrangement [O—Cu—Cl = 161.90 (15) and 151.94 (15)° for Cu4 and Cu6, respectively], but also one (for Cu4) and two (for Cu6) very long Cu—Cl contacts, which are right on the borderline of being considered as bonds.
In every case, the CuI atom also has two short Cu···Cu contacts to adjacent metal atoms with the separations indicated in the caption of Fig. 1; these are further discussed below. Bond-valence sums (Brown & Altermatt, 1985) for atoms Cu1 (1.05), Cu2 (1.04), Cu3 (1.03), Cu4 (1.01), Cu5 (1.05) and Cu6 (1.05) are in excellent agreement with the expected value of 1.00 for the cuprous ion, confirming that reduction from Cu2+ has occurred.
The other group of metal atoms, Cu7–Cu10 (Cu7 and Cu10 lie on crystallographic inversion centres) are bonded to two ligand N atoms (Cu—N < 2.00 Å) in a linear or near-linear geometry [N—Cu—N = 180, 177.8 (2), 176.6 (2) and 180° for Cu7, Cu8, Cu9 and Cu10, respectively]. Again, these atoms have a number of Cu···O and Cu···Cl contacts slightly shorter that the van der Waals radii sums noted above, with some of the former being just significant as bonds based on the Brown bond-valence criterion (Table 1). Even when the long Cu—O bonds are included, this group of CuI atoms appear to be substantially `under-bonded' (Saines et al., 2006), with their BVS values of 0.66 (Cu7), 0.74 (Cu8), 0.72 (Cu9) and 0.71 (Cu10) all significantly smaller than the expected value of 1.00. Considering just the Cu—N bonds and Cu···Cu contacts, each of atoms Cu7–Cu10 has an approximate `square-planar' trans-Cu2N2 coordination geometry.
The carboxylate group of the C1-containing benzoate ligand is slightly rotated from the plane of the aromatic ring, by 1.3 (5)°; the equivalent values for the for the C8- and C15-containing anions are much larger, at 18.4 (4) and 18.5 (4)°, respectively. The C—O carboxylate bond lengths indicate substantial delocalization in each case such that the two C—O bond lengths are almost equal (Table 2). For each ligand, the CuI atoms bonded to the O atoms are displaced in an opposite sense from the CO2 plane: for the C7/O1/O2 group, atoms Cu1 and Cu2 are displaced by -0.36 (2) and 0.44 (2) Å, respectively; for C14/O3/O4, Cu3 and Cu4 are displaced by -0.58 (2) and 0.44 (2) Å, respectively; for C21/O5/O6, Cu5 and Cu6 are displaced by -0.57 (2) and 0.48 (2) Å, respectively. Neglecting the borderline long Cu—O bonds, each benzoate ligand bonds to four nearby CuI atoms in a µ4N,N',O,O'-mode. The same µ4 ligand-bonding mode (but with a totally different overall structure) has been seen in [Mn(C7H7N2O2)(N3)] (Chen et al., 2009). Each chloride ion in (1) is bonded to two CuI atoms, with Cu—Cl < 2.5 Å and Cl—Cu—Cl < 125°, but a third metal atom is also present within 3.1 Å.
The packing in (1) is a dense three-dimensional polymeric network without any identifiable channels or voids. When viewed down the [101] direction (Fig. 3), chains of CuI atoms (involving Cu1–Cu6) and chloride ions are apparent, which are shown in more detail in Fig. 4, where four-, six- and eight-atom loops (Peng et al., 2010) are apparent in the chains. The packing is consolidated by N—H···Cl hydrogen bonds (Table 2), with all 12 N—H groups participating in such an interaction (mean H···Cl = 2.62 Å and mean N—H···Cl = 165°) Any possible aromatic π–π stacking in the crystal of (1) must be extremely weak, as the shortest separation of the centroids of the benzene rings is greater than 4.0 Å.
The Cu···Cu contacts (or bonds?) in (1) merit some further discussion. As might be expected, the shortest separations occur between pairs of metal atoms bridged by a benzoate ligand: this type of interaction has been termed `semi supported' by Schmidbaur & Schier (2012): the relevant data for (1) are Cu1···Cu2 = 2.7454 (13) Å, Cu3···Cu4 = 2.7350 (14) Å and Cu5···Cu6 = 2.7551 (15) Å. These separations are significantly shorter than those in the recently reported semi-supported (by one bulky benzoate ligand) linear polymer [Cu(C16H23O2)]n (Hietsoi et al., 2011), with Cu···Cu = 2.9397 (5) Å, but are much longer than the `fully supported' (by two bridging benzoate ligands) Cu···Cu distance of 2.493 Å (s.u. value not stated) in the dimeric compound [Cu2(C16H23O2)2(C6H4Cl2)2] (Batsanov, 2001).
The `unsupported' (no bridging ligands) Cu···Cu contacts in (1) between the CuI atoms associated with the benzoate ligands (Cu1–Cu6) and the CuI atoms showing a local linear N—Cu—N geometry (Cu7–Cu10) show considerable variation in their lengths, with the Cu4···Cu9 [2.8145 (12) Å] and Cu6···Cu10 [2.8455 (10) Å] separations being almost as short as the semi-supported bonds. Conversely, the Cu1···Cu7 [3.0169 (9) Å] and Cu2···Cu8 [3.0174 (12) Å] links are of intermediate length, and the Cu5···Cu9 [3.1067 (12) Å] and Cu3···Cu8 [3.2142 (13) Å] contacts are the longest. If all of these contacts, which are significantly shorter than twice the van der Waals radius of 1.92 Å for Cu+ according to Batsanov (2001), are regarded as cuprophilic Cu···Cu interactions/bonds, then infinite [101] zigzag chains of CuI atoms occur (Fig. 5). Yet another way to visualize the structure is in terms of the Cu+ and Cl- ions: when these are considered together, an unusual three-dimensional cationic open framework of stoichiometry [Cu3Cl2]+ results (including the Cu···Cu links), which surrounds [101] channels (Fig. 6).
The serendipitous redox synthesis and crystal structure of [Cu3(C7H7N2O2)Cl2] have been described. A wide variety of copper coordination polyhedra occur, including CuOCl2 (T-shape), CuOCl3 (tetrahedral) and CuN2 (linear), which obviously correlate with the different functional groups (carboxylate and amine) of the ligand. Every CuI atom has two near neighbours and if all these are regarded as cuprophilic bonds, then infinite zigzag Cu···Cu chains occur in the crystal.
Compounds containing copper(I) (electronic configuration [Ar]3d10) are of interest for their role in biological processes (Tsukihara et al., 1995) and the presence of short (< ~3.2 Å) Cu···Cu contacts in their crystal structures (Arkhireeva et al., 1990; Zheng et al., 2005; Sundararaman et al., 2005; Doshi et al., 2012, Cotton et al., 1998; Carvajal et al., 2004). The nature of these contacts has been debated, with some authors (Zheng et al., 2005; Sundararaman et al., 2005; Doshi et al., 2012) describing them as weakly attractive (bonding) `cuprophilic' interactions akin to the well-established argentophilic Ag···Ag (Barreiro et al., 2013) and aurophilic Au···Au (Schmidbaur & Schier, 2012) weak bonds in silver(I) and gold(I) compounds, respectively. However, other workers have questioned whether these Cu···Cu contacts represent bonds at all and suggested that the short metal–metal distances observed are either constrained by the ligand geometry (Cotton et al., 1998) or are largely ionic in nature (Carvajal et al., 2004).
Here, we describe the hydrothermal synthesis and crystal structure of the three-dimensional coordination polymer [Cu3(C7H7N2O2)Cl2]n, (1), which arose from the unexpected reduction of the CuCl2 starting material to Cu+ under basic conditions; C7H7N2O2- is the 3,5-diaminobenzoate anion.
The lack of colour of the crystals of (1) is consistent with the 3d10 electron configuration of the Cu+ ion (Doshi et al., 2012; Kappenstein & Hugel, 1978). The precise nature of the redox reaction that generated the cuprous ions is unknown, but similar reductions of Cu2+ → Cu+ in the presence of a nitrogen-containing ligand under hydrothermal conditions have been reported by other workers (Xin et al., 2013) and indeed this type of reduction has been described as a `recommended route' (Peng et al., 2010) to cuprous–halide compounds from Cu2+-containing starting materials. The asymmetric unit of (1), which contains no fewer than ten crystallographically independent CuI atoms (eight of which lie on general positions and two on crystallographic inversion centres), as well as three benzoate ligands and six chloride ions, is shown in Fig. 1.
The copper coordination geometries in (1) can be split into several distinct types. Atoms Cu1–Cu6 are bonded to various combinations of benzoate O atoms and chloride ions (Table 1). In this complex structure, all these CuI atoms are surrounded by a number of O and Cl atoms at distances around or slightly shorter that the Bondi (1964) van der Waals radii sum of Cu···O = 2.92 Å and Cu···Cl = 3.15 Å, but by using the Brown criterion of 0.04 of a bond-valence unit to indicate a significant chemical bond to a univalent cation (Brown, 2002), the following conclusions can be drawn: Cu1 and Cu2 are bonded to a benzoate O atom and two chloride ions in a distorted T-shape, whereas Cu3 and Cu5 are bonded to one O atom and three chloride ions in very distorted tetrahedral arrangements (Fig. 2). The Cu4 and Cu6 coordination geometries are ambiguous; they have two well-defined short bonds to one O and one chloride ion in an approximately linear arrangement [O—Cu—Cl = 161.90 (15) and 151.94 (15)° for Cu4 and Cu6, respectively], but also one (for Cu4) and two (for Cu6) very long Cu—Cl contacts, which are right on the borderline of being considered as bonds.
In every case, the CuI atom also has two short Cu···Cu contacts to adjacent metal atoms with the separations indicated in the caption of Fig. 1; these are further discussed below. Bond-valence sums (Brown & Altermatt, 1985) for atoms Cu1 (1.05), Cu2 (1.04), Cu3 (1.03), Cu4 (1.01), Cu5 (1.05) and Cu6 (1.05) are in excellent agreement with the expected value of 1.00 for the cuprous ion, confirming that reduction from Cu2+ has occurred.
The other group of metal atoms, Cu7–Cu10 (Cu7 and Cu10 lie on crystallographic inversion centres) are bonded to two ligand N atoms (Cu—N < 2.00 Å) in a linear or near-linear geometry [N—Cu—N = 180, 177.8 (2), 176.6 (2) and 180° for Cu7, Cu8, Cu9 and Cu10, respectively]. Again, these atoms have a number of Cu···O and Cu···Cl contacts slightly shorter that the van der Waals radii sums noted above, with some of the former being just significant as bonds based on the Brown bond-valence criterion (Table 1). Even when the long Cu—O bonds are included, this group of CuI atoms appear to be substantially `under-bonded' (Saines et al., 2006), with their BVS values of 0.66 (Cu7), 0.74 (Cu8), 0.72 (Cu9) and 0.71 (Cu10) all significantly smaller than the expected value of 1.00. Considering just the Cu—N bonds and Cu···Cu contacts, each of atoms Cu7–Cu10 has an approximate `square-planar' trans-Cu2N2 coordination geometry.
The carboxylate group of the C1-containing benzoate ligand is slightly rotated from the plane of the aromatic ring, by 1.3 (5)°; the equivalent values for the for the C8- and C15-containing anions are much larger, at 18.4 (4) and 18.5 (4)°, respectively. The C—O carboxylate bond lengths indicate substantial delocalization in each case such that the two C—O bond lengths are almost equal (Table 2). For each ligand, the CuI atoms bonded to the O atoms are displaced in an opposite sense from the CO2 plane: for the C7/O1/O2 group, atoms Cu1 and Cu2 are displaced by -0.36 (2) and 0.44 (2) Å, respectively; for C14/O3/O4, Cu3 and Cu4 are displaced by -0.58 (2) and 0.44 (2) Å, respectively; for C21/O5/O6, Cu5 and Cu6 are displaced by -0.57 (2) and 0.48 (2) Å, respectively. Neglecting the borderline long Cu—O bonds, each benzoate ligand bonds to four nearby CuI atoms in a µ4N,N',O,O'-mode. The same µ4 ligand-bonding mode (but with a totally different overall structure) has been seen in [Mn(C7H7N2O2)(N3)] (Chen et al., 2009). Each chloride ion in (1) is bonded to two CuI atoms, with Cu—Cl < 2.5 Å and Cl—Cu—Cl < 125°, but a third metal atom is also present within 3.1 Å.
The packing in (1) is a dense three-dimensional polymeric network without any identifiable channels or voids. When viewed down the [101] direction (Fig. 3), chains of CuI atoms (involving Cu1–Cu6) and chloride ions are apparent, which are shown in more detail in Fig. 4, where four-, six- and eight-atom loops (Peng et al., 2010) are apparent in the chains. The packing is consolidated by N—H···Cl hydrogen bonds (Table 2), with all 12 N—H groups participating in such an interaction (mean H···Cl = 2.62 Å and mean N—H···Cl = 165°) Any possible aromatic π–π stacking in the crystal of (1) must be extremely weak, as the shortest separation of the centroids of the benzene rings is greater than 4.0 Å.
The Cu···Cu contacts (or bonds?) in (1) merit some further discussion. As might be expected, the shortest separations occur between pairs of metal atoms bridged by a benzoate ligand: this type of interaction has been termed `semi supported' by Schmidbaur & Schier (2012): the relevant data for (1) are Cu1···Cu2 = 2.7454 (13) Å, Cu3···Cu4 = 2.7350 (14) Å and Cu5···Cu6 = 2.7551 (15) Å. These separations are significantly shorter than those in the recently reported semi-supported (by one bulky benzoate ligand) linear polymer [Cu(C16H23O2)]n (Hietsoi et al., 2011), with Cu···Cu = 2.9397 (5) Å, but are much longer than the `fully supported' (by two bridging benzoate ligands) Cu···Cu distance of 2.493 Å (s.u. value not stated) in the dimeric compound [Cu2(C16H23O2)2(C6H4Cl2)2] (Batsanov, 2001).
The `unsupported' (no bridging ligands) Cu···Cu contacts in (1) between the CuI atoms associated with the benzoate ligands (Cu1–Cu6) and the CuI atoms showing a local linear N—Cu—N geometry (Cu7–Cu10) show considerable variation in their lengths, with the Cu4···Cu9 [2.8145 (12) Å] and Cu6···Cu10 [2.8455 (10) Å] separations being almost as short as the semi-supported bonds. Conversely, the Cu1···Cu7 [3.0169 (9) Å] and Cu2···Cu8 [3.0174 (12) Å] links are of intermediate length, and the Cu5···Cu9 [3.1067 (12) Å] and Cu3···Cu8 [3.2142 (13) Å] contacts are the longest. If all of these contacts, which are significantly shorter than twice the van der Waals radius of 1.92 Å for Cu+ according to Batsanov (2001), are regarded as cuprophilic Cu···Cu interactions/bonds, then infinite [101] zigzag chains of CuI atoms occur (Fig. 5). Yet another way to visualize the structure is in terms of the Cu+ and Cl- ions: when these are considered together, an unusual three-dimensional cationic open framework of stoichiometry [Cu3Cl2]+ results (including the Cu···Cu links), which surrounds [101] channels (Fig. 6).
The serendipitous redox synthesis and crystal structure of [Cu3(C7H7N2O2)Cl2] have been described. A wide variety of copper coordination polyhedra occur, including CuOCl2 (T-shape), CuOCl3 (tetrahedral) and CuN2 (linear), which obviously correlate with the different functional groups (carboxylate and amine) of the ligand. Every CuI atom has two near neighbours and if all these are regarded as cuprophilic bonds, then infinite zigzag Cu···Cu chains occur in the crystal.
A mixture of CuCl2 (134 mg, 1.0 mmol) and 3,5-diaminobenzoic acid (304 mg, 2.0 mmol) was added to 1 M KOH (2.0 ml) with stirring to yield a pale-blue solution. This mixture was heated to 423 K in a 23 ml Teflon-lined autoclave for 15 h and then cooled to room temperature over a period of several hours. Colourless blocks of (1) were recovered from the reaction by filtration and rinsing with water and acetone.
Crystal data, data collection and structure refinement details are summarized in Table 1. The crystal quality was only fair, which may correlate with the rather high residuals and the larger than expected difference-map features (Δρ min. max. = -2.7 and 3.4 e Å-3, respectively). The H atoms were placed geometrically (C—H = 0.95 Å and N—H = 0.92 Å) and refined as riding atoms, with the constraint Uiso(H) = Ueq(carrier). This structure has a strong subcell (C2/c, a = 13.077, b = 10.894, c = 7.209 Å, β = 94.64° and V = 1023.6 Å3), but refinements in this space group, in which the asymmetric unit contains two CuI atoms (one with symmetry 1), two Cl atoms and half a ligand molecule, led to much higher residuals [R(F) ~0.20], large difference Fourier peaks (~±6 e Å-3) in the vicinity of the CuI atoms and many systematic absence violations and was therefore rejected. The structural models were analysed and verified with PLATON (Spek, 2009).
Data collection: APEX2 (Bruker, 2004); cell refinement: SAINT (Bruker, 2004); data reduction: SAINT (Bruker, 2004); program(s) used to solve structure: SHELXS97 (Sheldrick, 2008); program(s) used to refine structure: SHELXL2014 (Sheldrick, 2015); molecular graphics: ORTEP-3 for Windows (Farrugia, 2012) and ATOMS (Dowty, 2004); software used to prepare material for publication: SHELXL2014 (Sheldrick, 2015).
| [Cu3(C7H7CN2O2)Cl2] | F(000) = 2400 |
| Mr = 412.67 | Dx = 2.677 Mg m−3 |
| Monoclinic, P21/n | Mo Kα radiation, λ = 0.71073 Å |
| a = 14.4127 (2) Å | Cell parameters from 23785 reflections |
| b = 10.8942 (2) Å | θ = 1.0–30.0° |
| c = 20.2348 (4) Å | µ = 6.68 mm−1 |
| β = 104.8395 (7)° | T = 180 K |
| V = 3071.20 (9) Å3 | Block, colourless |
| Z = 12 | 0.39 × 0.16 × 0.14 mm |
| Bruker APEXII CCD diffractometer | 4798 reflections with I > 2σ(I) |
| ω scans | Rint = 0.069 |
| Absorption correction: multi-scan (SADABS; Bruker, 2004) | θmax = 26.0°, θmin = 2.1° |
| Tmin = 0.181, Tmax = 0.455 | h = −17→17 |
| 16907 measured reflections | k = −12→13 |
| 5987 independent reflections | l = −22→24 |
| Refinement on F2 | 0 restraints |
| Least-squares matrix: full | Hydrogen site location: inferred from neighbouring sites |
| R[F2 > 2σ(F2)] = 0.069 | H-atom parameters constrained |
| wR(F2) = 0.202 | w = 1/[σ2(Fo2) + (0.1163P)2 + 23.2868P] where P = (Fo2 + 2Fc2)/3 |
| S = 1.04 | (Δ/σ)max = 0.001 |
| 5987 reflections | Δρmax = 3.40 e Å−3 |
| 436 parameters | Δρmin = −2.71 e Å−3 |
| [Cu3(C7H7CN2O2)Cl2] | V = 3071.20 (9) Å3 |
| Mr = 412.67 | Z = 12 |
| Monoclinic, P21/n | Mo Kα radiation |
| a = 14.4127 (2) Å | µ = 6.68 mm−1 |
| b = 10.8942 (2) Å | T = 180 K |
| c = 20.2348 (4) Å | 0.39 × 0.16 × 0.14 mm |
| β = 104.8395 (7)° |
| Bruker APEXII CCD diffractometer | 5987 independent reflections |
| Absorption correction: multi-scan (SADABS; Bruker, 2004) | 4798 reflections with I > 2σ(I) |
| Tmin = 0.181, Tmax = 0.455 | Rint = 0.069 |
| 16907 measured reflections |
| R[F2 > 2σ(F2)] = 0.069 | 0 restraints |
| wR(F2) = 0.202 | H-atom parameters constrained |
| S = 1.04 | w = 1/[σ2(Fo2) + (0.1163P)2 + 23.2868P] where P = (Fo2 + 2Fc2)/3 |
| 5987 reflections | Δρmax = 3.40 e Å−3 |
| 436 parameters | Δρmin = −2.71 e Å−3 |
Geometry. All esds (except the esd in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell esds are taken into account individually in the estimation of esds in distances, angles and torsion angles; correlations between esds in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell esds is used for estimating esds involving l.s. planes. |
| x | y | z | Uiso*/Ueq | ||
| Cu1 | 0.46131 (6) | −0.15788 (7) | 0.11250 (4) | 0.0217 (2) | |
| Cu2 | 0.37595 (6) | −0.15297 (7) | 0.21918 (5) | 0.0228 (2) | |
| Cu3 | 0.26100 (8) | 0.15557 (9) | 0.44481 (5) | 0.0351 (3) | |
| Cu4 | 0.25123 (7) | 0.12385 (8) | 0.57694 (5) | 0.0286 (3) | |
| Cu5 | 0.08697 (7) | −0.14980 (8) | 0.77262 (5) | 0.0325 (3) | |
| Cu6 | 0.08418 (7) | −0.12935 (8) | 0.90781 (5) | 0.0275 (3) | |
| Cu7 | 0.5000 | 0.0000 | 0.0000 | 0.0177 (3) | |
| Cu8 | 0.32887 (5) | −0.00559 (7) | 0.33262 (4) | 0.0199 (2) | |
| Cu9 | 0.17070 (5) | 0.00558 (7) | 0.67278 (4) | 0.0206 (2) | |
| Cu10 | 0.0000 | 0.0000 | 1.0000 | 0.0205 (3) | |
| C1 | 0.4207 (4) | 0.2083 (6) | 0.1627 (3) | 0.0097 (12) | |
| C2 | 0.4815 (4) | 0.2722 (5) | 0.1312 (3) | 0.0104 (11) | |
| H2 | 0.5227 | 0.2291 | 0.1094 | 0.013* | |
| C3 | 0.4813 (4) | 0.3997 (5) | 0.1322 (3) | 0.0106 (11) | |
| C4 | 0.4220 (4) | 0.4639 (6) | 0.1635 (3) | 0.0113 (12) | |
| H4 | 0.4218 | 0.5511 | 0.1634 | 0.014* | |
| C5 | 0.3622 (4) | 0.3990 (5) | 0.1954 (3) | 0.0107 (11) | |
| C6 | 0.3601 (4) | 0.2720 (5) | 0.1953 (3) | 0.0096 (11) | |
| H6 | 0.3185 | 0.2288 | 0.2168 | 0.012* | |
| C7 | 0.4201 (4) | 0.0721 (6) | 0.1633 (3) | 0.0110 (12) | |
| N1 | 0.5492 (4) | 0.4670 (5) | 0.1044 (3) | 0.0145 (10) | |
| H1A | 0.5233 | 0.5407 | 0.0883 | 0.017* | |
| H1B | 0.5606 | 0.4243 | 0.0686 | 0.017* | |
| N2 | 0.2977 (4) | 0.4666 (5) | 0.2273 (3) | 0.0147 (11) | |
| H2A | 0.3248 | 0.5405 | 0.2419 | 0.018* | |
| H2B | 0.2915 | 0.4243 | 0.2647 | 0.018* | |
| C8 | 0.2549 (4) | −0.2226 (5) | 0.4944 (3) | 0.0092 (11) | |
| C9 | 0.3132 (4) | −0.2847 (5) | 0.4599 (3) | 0.0104 (11) | |
| H9 | 0.3537 | −0.2406 | 0.4379 | 0.013* | |
| C10 | 0.3110 (4) | −0.4128 (5) | 0.4583 (3) | 0.0106 (11) | |
| C11 | 0.2500 (4) | −0.4779 (5) | 0.4887 (3) | 0.0109 (11) | |
| H11 | 0.2471 | −0.5649 | 0.4857 | 0.013* | |
| C12 | 0.1938 (4) | −0.4154 (5) | 0.5231 (3) | 0.0095 (11) | |
| C13 | 0.1960 (4) | −0.2881 (5) | 0.5268 (3) | 0.0112 (11) | |
| H13 | 0.1577 | −0.2459 | 0.5514 | 0.013* | |
| C14 | 0.2570 (4) | −0.0856 (5) | 0.4979 (3) | 0.0099 (11) | |
| N3 | 0.3797 (4) | −0.4791 (5) | 0.4304 (3) | 0.0142 (11) | |
| H3A | 0.3906 | −0.4368 | 0.3943 | 0.017* | |
| H3B | 0.3554 | −0.5539 | 0.4152 | 0.017* | |
| N4 | 0.1299 (4) | −0.4819 (5) | 0.5553 (3) | 0.0134 (10) | |
| H4A | 0.1282 | −0.4423 | 0.5946 | 0.016* | |
| H4B | 0.1548 | −0.5580 | 0.5670 | 0.016* | |
| C15 | 0.0841 (3) | 0.2246 (6) | 0.8298 (3) | 0.0093 (12) | |
| C16 | 0.1397 (4) | 0.2878 (5) | 0.7943 (3) | 0.0093 (11) | |
| H16 | 0.1787 | 0.2441 | 0.7710 | 0.011* | |
| C17 | 0.1380 (4) | 0.4154 (5) | 0.7931 (3) | 0.0106 (11) | |
| C18 | 0.0782 (4) | 0.4802 (6) | 0.8243 (3) | 0.0100 (12) | |
| H18 | 0.0752 | 0.5672 | 0.8217 | 0.012* | |
| C19 | 0.0224 (4) | 0.4157 (5) | 0.8595 (3) | 0.0101 (11) | |
| C20 | 0.0262 (4) | 0.2895 (5) | 0.8635 (3) | 0.0102 (11) | |
| H20 | −0.0103 | 0.2467 | 0.8891 | 0.012* | |
| C21 | 0.0866 (3) | 0.0876 (6) | 0.8327 (3) | 0.0089 (12) | |
| N5 | 0.2042 (4) | 0.4809 (5) | 0.7630 (3) | 0.0146 (11) | |
| H5A | 0.1786 | 0.5553 | 0.7479 | 0.018* | |
| H5B | 0.2121 | 0.4382 | 0.7262 | 0.018* | |
| N6 | −0.0426 (4) | 0.4826 (5) | 0.8907 (3) | 0.0142 (11) | |
| H6A | −0.0168 | 0.5576 | 0.9039 | 0.017* | |
| H6B | −0.0469 | 0.4417 | 0.9290 | 0.017* | |
| O1 | 0.4743 (3) | 0.0166 (4) | 0.1325 (2) | 0.0180 (10) | |
| O2 | 0.3656 (3) | 0.0181 (4) | 0.1938 (2) | 0.0206 (10) | |
| O3 | 0.2921 (4) | −0.0264 (4) | 0.4563 (2) | 0.0199 (10) | |
| O4 | 0.2252 (4) | −0.0391 (4) | 0.5446 (2) | 0.0216 (10) | |
| O5 | 0.1191 (3) | 0.0303 (4) | 0.7893 (2) | 0.0167 (9) | |
| O6 | 0.0561 (3) | 0.0371 (4) | 0.8790 (2) | 0.0172 (9) | |
| Cl1 | 0.39320 (11) | −0.29137 (15) | 0.03396 (8) | 0.0225 (4) | |
| Cl2 | 0.43913 (10) | −0.29010 (13) | 0.29733 (8) | 0.0172 (3) | |
| Cl3 | 0.27191 (12) | 0.25776 (14) | 0.34882 (8) | 0.0214 (4) | |
| Cl4 | 0.26239 (11) | 0.28564 (14) | 0.63859 (8) | 0.0202 (4) | |
| Cl5 | 0.10718 (11) | −0.25623 (14) | 0.68104 (7) | 0.0186 (3) | |
| Cl6 | 0.08403 (13) | −0.28007 (15) | 0.97701 (8) | 0.0267 (4) |
| U11 | U22 | U33 | U12 | U13 | U23 | |
| Cu1 | 0.0264 (5) | 0.0164 (4) | 0.0250 (5) | −0.0048 (3) | 0.0116 (4) | −0.0076 (3) |
| Cu2 | 0.0257 (5) | 0.0185 (4) | 0.0285 (5) | 0.0069 (3) | 0.0145 (4) | 0.0101 (3) |
| Cu3 | 0.0522 (7) | 0.0215 (5) | 0.0343 (6) | 0.0021 (4) | 0.0159 (5) | 0.0124 (4) |
| Cu4 | 0.0384 (6) | 0.0200 (5) | 0.0280 (5) | −0.0033 (4) | 0.0099 (4) | −0.0105 (4) |
| Cu5 | 0.0354 (6) | 0.0252 (5) | 0.0399 (6) | −0.0039 (4) | 0.0151 (5) | −0.0167 (4) |
| Cu6 | 0.0326 (5) | 0.0203 (5) | 0.0271 (5) | −0.0019 (4) | 0.0027 (4) | 0.0122 (4) |
| Cu7 | 0.0091 (6) | 0.0185 (6) | 0.0275 (6) | 0.0053 (4) | 0.0085 (5) | 0.0021 (5) |
| Cu8 | 0.0108 (5) | 0.0220 (5) | 0.0294 (5) | −0.0067 (3) | 0.0096 (4) | −0.0035 (3) |
| Cu9 | 0.0117 (5) | 0.0254 (5) | 0.0267 (5) | 0.0080 (3) | 0.0084 (4) | 0.0065 (3) |
| Cu10 | 0.0109 (6) | 0.0262 (6) | 0.0254 (6) | −0.0074 (4) | 0.0066 (5) | −0.0058 (5) |
| C1 | 0.007 (3) | 0.011 (3) | 0.008 (3) | 0.0022 (19) | −0.002 (2) | 0.000 (2) |
| C2 | 0.005 (2) | 0.017 (3) | 0.009 (3) | 0.001 (2) | 0.001 (2) | 0.000 (2) |
| C3 | 0.008 (3) | 0.013 (3) | 0.010 (3) | −0.004 (2) | 0.000 (2) | 0.001 (2) |
| C4 | 0.009 (3) | 0.013 (3) | 0.012 (3) | −0.004 (2) | 0.002 (2) | 0.000 (2) |
| C5 | 0.006 (3) | 0.014 (3) | 0.009 (3) | 0.000 (2) | −0.003 (2) | −0.004 (2) |
| C6 | 0.007 (3) | 0.013 (3) | 0.008 (3) | −0.005 (2) | 0.001 (2) | −0.003 (2) |
| C7 | 0.009 (3) | 0.013 (3) | 0.011 (3) | 0.002 (2) | 0.002 (2) | −0.002 (2) |
| N1 | 0.010 (2) | 0.016 (2) | 0.020 (3) | −0.003 (2) | 0.008 (2) | 0.004 (2) |
| N2 | 0.017 (3) | 0.009 (2) | 0.023 (3) | 0.000 (2) | 0.012 (2) | −0.002 (2) |
| C8 | 0.005 (2) | 0.012 (3) | 0.010 (3) | 0.001 (2) | −0.001 (2) | 0.000 (2) |
| C9 | 0.005 (3) | 0.013 (3) | 0.013 (3) | −0.003 (2) | 0.003 (2) | −0.001 (2) |
| C10 | 0.008 (3) | 0.012 (3) | 0.011 (3) | 0.005 (2) | 0.001 (2) | −0.004 (2) |
| C11 | 0.008 (3) | 0.009 (3) | 0.016 (3) | 0.002 (2) | 0.002 (2) | 0.003 (2) |
| C12 | 0.005 (2) | 0.009 (3) | 0.014 (3) | −0.002 (2) | 0.001 (2) | 0.003 (2) |
| C13 | 0.012 (3) | 0.009 (3) | 0.013 (3) | 0.003 (2) | 0.004 (2) | −0.002 (2) |
| C14 | 0.004 (2) | 0.012 (3) | 0.012 (3) | −0.002 (2) | −0.001 (2) | 0.000 (2) |
| N3 | 0.014 (3) | 0.011 (2) | 0.020 (3) | 0.002 (2) | 0.008 (2) | −0.004 (2) |
| N4 | 0.015 (3) | 0.010 (2) | 0.017 (3) | −0.0028 (19) | 0.008 (2) | 0.002 (2) |
| C15 | 0.002 (3) | 0.013 (3) | 0.012 (3) | 0.0006 (19) | 0.000 (2) | 0.001 (2) |
| C16 | 0.008 (3) | 0.009 (3) | 0.011 (3) | 0.001 (2) | 0.003 (2) | 0.002 (2) |
| C17 | 0.005 (2) | 0.010 (3) | 0.016 (3) | 0.000 (2) | 0.001 (2) | 0.002 (2) |
| C18 | 0.006 (3) | 0.010 (3) | 0.014 (3) | 0.0021 (19) | 0.003 (2) | 0.002 (2) |
| C19 | 0.004 (2) | 0.011 (3) | 0.014 (3) | −0.001 (2) | 0.001 (2) | −0.006 (2) |
| C20 | 0.009 (3) | 0.011 (3) | 0.012 (3) | 0.000 (2) | 0.005 (2) | −0.003 (2) |
| C21 | 0.005 (3) | 0.011 (3) | 0.011 (3) | 0.0014 (18) | 0.001 (2) | 0.000 (2) |
| N5 | 0.017 (3) | 0.012 (2) | 0.018 (3) | −0.003 (2) | 0.009 (2) | 0.002 (2) |
| N6 | 0.015 (3) | 0.011 (2) | 0.021 (3) | 0.0056 (19) | 0.013 (2) | 0.000 (2) |
| O1 | 0.018 (2) | 0.013 (2) | 0.026 (2) | 0.0008 (17) | 0.0106 (19) | −0.0006 (18) |
| O2 | 0.023 (2) | 0.014 (2) | 0.028 (3) | 0.0032 (18) | 0.013 (2) | 0.0036 (19) |
| O3 | 0.027 (3) | 0.012 (2) | 0.026 (2) | 0.0021 (18) | 0.018 (2) | 0.0005 (19) |
| O4 | 0.034 (3) | 0.015 (2) | 0.022 (2) | −0.002 (2) | 0.018 (2) | −0.0061 (19) |
| O5 | 0.023 (2) | 0.011 (2) | 0.020 (2) | −0.0025 (18) | 0.0128 (19) | −0.0040 (18) |
| O6 | 0.027 (2) | 0.011 (2) | 0.019 (2) | 0.0030 (18) | 0.0140 (19) | 0.0021 (17) |
| Cl1 | 0.0208 (8) | 0.0233 (8) | 0.0202 (8) | −0.0011 (6) | −0.0007 (6) | −0.0058 (6) |
| Cl2 | 0.0145 (7) | 0.0169 (7) | 0.0179 (7) | 0.0024 (5) | 0.0000 (6) | 0.0044 (6) |
| Cl3 | 0.0226 (8) | 0.0234 (8) | 0.0159 (7) | 0.0000 (6) | 0.0008 (6) | 0.0040 (6) |
| Cl4 | 0.0204 (8) | 0.0179 (7) | 0.0178 (7) | 0.0031 (6) | −0.0031 (6) | −0.0070 (6) |
| Cl5 | 0.0181 (7) | 0.0209 (7) | 0.0147 (7) | 0.0019 (6) | 0.0005 (6) | −0.0047 (6) |
| Cl6 | 0.0313 (9) | 0.0233 (8) | 0.0182 (8) | −0.0106 (7) | −0.0070 (7) | 0.0095 (6) |
| Cu1—O1 | 1.943 (4) | C7—O2 | 1.261 (7) |
| Cu1—Cl1 | 2.1921 (17) | C7—O1 | 1.270 (7) |
| Cu1—Cl5i | 2.3928 (17) | N1—Cu9viii | 1.957 (5) |
| Cu1—Cu2 | 2.7454 (13) | N1—H1A | 0.9200 |
| Cu1—Cu7 | 3.0169 (9) | N1—H1B | 0.9200 |
| Cu2—O2 | 1.929 (5) | N2—Cu8iii | 1.938 (5) |
| Cu2—Cl2 | 2.1964 (16) | N2—H2A | 0.9200 |
| Cu2—Cl3ii | 2.4246 (18) | N2—H2B | 0.9200 |
| Cu2—Cu8 | 3.0174 (12) | C8—C13 | 1.395 (8) |
| Cu3—O3 | 2.033 (5) | C8—C9 | 1.398 (8) |
| Cu3—Cl3 | 2.2788 (18) | C8—C14 | 1.494 (8) |
| Cu3—Cl1iii | 2.439 (2) | C9—C10 | 1.395 (8) |
| Cu3—Cl6iv | 2.485 (2) | C9—H9 | 0.9500 |
| Cu3—Cu4 | 2.7350 (14) | C10—C11 | 1.390 (8) |
| Cu4—O4 | 1.896 (5) | C10—N3 | 1.453 (7) |
| Cu4—Cl4 | 2.1415 (17) | C11—C12 | 1.376 (8) |
| Cu4—Cl1iii | 2.7981 (19) | C11—H11 | 0.9500 |
| Cu4—Cu9 | 2.8145 (12) | C12—C13 | 1.389 (8) |
| Cu5—O5 | 2.024 (4) | C12—N4 | 1.452 (7) |
| Cu5—Cl5 | 2.2672 (17) | C13—H13 | 0.9500 |
| Cu5—Cl2v | 2.4009 (18) | C14—O4 | 1.257 (7) |
| Cu5—Cl4vi | 2.5373 (18) | C14—O3 | 1.263 (7) |
| Cu5—Cu6 | 2.7551 (15) | N3—Cu10vi | 1.946 (5) |
| Cu6—O6 | 1.916 (4) | N3—H3A | 0.9200 |
| Cu6—Cl6 | 2.1583 (18) | N3—H3B | 0.9200 |
| Cu6—Cl4vi | 2.7735 (19) | N4—Cu7ii | 1.929 (5) |
| Cu6—Cl2v | 2.7828 (18) | N4—H4A | 0.9200 |
| Cu6—Cu10 | 2.8455 (10) | N4—H4B | 0.9200 |
| Cu7—N4i | 1.929 (5) | C15—C16 | 1.387 (8) |
| Cu7—N4iii | 1.929 (5) | C15—C20 | 1.398 (8) |
| Cu7—Cu1vii | 3.0168 (9) | C15—C21 | 1.494 (9) |
| Cu8—N2ii | 1.938 (5) | C16—C17 | 1.391 (8) |
| Cu8—N6viii | 1.939 (6) | C16—H16 | 0.9500 |
| Cu8—O3 | 2.697 (4) | C17—C18 | 1.385 (8) |
| Cu9—N5vi | 1.953 (5) | C17—N5 | 1.444 (7) |
| Cu9—N1ix | 1.957 (5) | C18—C19 | 1.394 (8) |
| Cu9—O5 | 2.661 (4) | C18—H18 | 0.9500 |
| Cu10—N3v | 1.946 (5) | C19—C20 | 1.377 (8) |
| Cu10—N3iv | 1.946 (5) | C19—N6 | 1.453 (7) |
| Cu10—O6 | 2.798 (4) | C20—H20 | 0.9500 |
| Cu10—O6x | 2.798 (4) | C21—O6 | 1.258 (7) |
| Cu10—Cu6x | 2.8455 (10) | C21—O5 | 1.260 (7) |
| C1—C2 | 1.395 (8) | N5—Cu9iv | 1.953 (5) |
| C1—C6 | 1.406 (8) | N5—H5A | 0.9200 |
| C1—C7 | 1.484 (9) | N5—H5B | 0.9200 |
| C2—C3 | 1.389 (8) | N6—Cu8ix | 1.939 (6) |
| C2—H2 | 0.9500 | N6—H6A | 0.9200 |
| C3—C4 | 1.378 (8) | N6—H6B | 0.9200 |
| C3—N1 | 1.448 (7) | Cl1—Cu3ii | 2.439 (2) |
| C4—C5 | 1.394 (8) | Cl2—Cu5i | 2.4010 (18) |
| C4—H4 | 0.9500 | Cl3—Cu2iii | 2.4246 (18) |
| C5—C6 | 1.384 (8) | Cl4—Cu5iv | 2.5373 (18) |
| C5—N2 | 1.458 (7) | Cl5—Cu1v | 2.3929 (17) |
| C6—H6 | 0.9500 | Cl6—Cu3vi | 2.485 (2) |
| O1—Cu1—Cl1 | 143.31 (15) | C10—C11—H11 | 120.3 |
| O1—Cu1—Cl5i | 103.78 (15) | C11—C12—C13 | 120.9 (5) |
| Cl1—Cu1—Cl5i | 108.94 (6) | C11—C12—N4 | 120.3 (5) |
| O2—Cu2—Cl2 | 147.00 (16) | C13—C12—N4 | 118.8 (5) |
| O2—Cu2—Cl3ii | 103.86 (15) | C12—C13—C8 | 119.6 (5) |
| Cl2—Cu2—Cl3ii | 106.14 (6) | C12—C13—H13 | 120.2 |
| O3—Cu3—Cl3 | 120.86 (15) | C8—C13—H13 | 120.2 |
| O3—Cu3—Cl1iii | 113.26 (14) | O4—C14—O3 | 125.4 (6) |
| Cl3—Cu3—Cl1iii | 107.42 (7) | O4—C14—C8 | 115.5 (5) |
| O3—Cu3—Cl6iv | 93.82 (16) | O3—C14—C8 | 119.0 (5) |
| Cl3—Cu3—Cl6iv | 99.08 (7) | C10—N3—Cu10vi | 110.3 (4) |
| Cl1iii—Cu3—Cl6iv | 122.15 (7) | C10—N3—H3A | 109.6 |
| O4—Cu4—Cl4 | 161.90 (15) | Cu10vi—N3—H3A | 109.6 |
| O5—Cu5—Cl5 | 124.08 (14) | C10—N3—H3B | 109.6 |
| O5—Cu5—Cl2v | 113.99 (14) | Cu10vi—N3—H3B | 109.6 |
| Cl5—Cu5—Cl2v | 109.72 (7) | H3A—N3—H3B | 108.1 |
| O5—Cu5—Cl4vi | 91.90 (14) | C12—N4—Cu7ii | 115.0 (4) |
| Cl5—Cu5—Cl4vi | 99.86 (6) | C12—N4—H4A | 108.5 |
| Cl2v—Cu5—Cl4vi | 114.90 (7) | Cu7ii—N4—H4A | 108.5 |
| O6—Cu6—Cl6 | 151.94 (15) | C12—N4—H4B | 108.5 |
| N4i—Cu7—N4iii | 180.0 | Cu7ii—N4—H4B | 108.5 |
| N2ii—Cu8—N6viii | 177.8 (2) | H4A—N4—H4B | 107.5 |
| N5vi—Cu9—N1ix | 176.6 (2) | C16—C15—C20 | 119.9 (6) |
| N3v—Cu10—N3iv | 180.0 (3) | C16—C15—C21 | 120.3 (5) |
| C2—C1—C6 | 120.4 (6) | C20—C15—C21 | 119.8 (5) |
| C2—C1—C7 | 120.6 (5) | C15—C16—C17 | 119.7 (5) |
| C6—C1—C7 | 119.0 (5) | C15—C16—H16 | 120.2 |
| C3—C2—C1 | 119.2 (5) | C17—C16—H16 | 120.2 |
| C3—C2—H2 | 120.4 | C18—C17—C16 | 120.8 (5) |
| C1—C2—H2 | 120.4 | C18—C17—N5 | 119.7 (5) |
| C4—C3—C2 | 121.2 (5) | C16—C17—N5 | 119.4 (5) |
| C4—C3—N1 | 118.9 (5) | C17—C18—C19 | 119.0 (6) |
| C2—C3—N1 | 119.7 (5) | C17—C18—H18 | 120.5 |
| C3—C4—C5 | 119.0 (6) | C19—C18—H18 | 120.5 |
| C3—C4—H4 | 120.5 | C20—C19—C18 | 120.9 (5) |
| C5—C4—H4 | 120.5 | C20—C19—N6 | 119.7 (5) |
| C6—C5—C4 | 121.4 (5) | C18—C19—N6 | 119.3 (5) |
| C6—C5—N2 | 119.3 (5) | C19—C20—C15 | 119.7 (5) |
| C4—C5—N2 | 119.2 (5) | C19—C20—H20 | 120.2 |
| C5—C6—C1 | 118.6 (5) | C15—C20—H20 | 120.2 |
| C5—C6—H6 | 120.7 | O6—C21—O5 | 124.4 (6) |
| C1—C6—H6 | 120.7 | O6—C21—C15 | 117.2 (5) |
| O2—C7—O1 | 123.7 (6) | O5—C21—C15 | 118.4 (5) |
| O2—C7—C1 | 118.5 (5) | C17—N5—Cu9iv | 112.6 (4) |
| O1—C7—C1 | 117.8 (5) | C17—N5—H5A | 109.1 |
| C3—N1—Cu9viii | 111.9 (4) | Cu9iv—N5—H5A | 109.1 |
| C3—N1—H1A | 109.2 | C17—N5—H5B | 109.1 |
| Cu9viii—N1—H1A | 109.2 | Cu9iv—N5—H5B | 109.1 |
| C3—N1—H1B | 109.2 | H5A—N5—H5B | 107.8 |
| Cu9viii—N1—H1B | 109.2 | C19—N6—Cu8ix | 115.1 (4) |
| H1A—N1—H1B | 107.9 | C19—N6—H6A | 108.5 |
| C5—N2—Cu8iii | 114.0 (4) | Cu8ix—N6—H6A | 108.5 |
| C5—N2—H2A | 108.8 | C19—N6—H6B | 108.5 |
| Cu8iii—N2—H2A | 108.8 | Cu8ix—N6—H6B | 108.5 |
| C5—N2—H2B | 108.8 | H6A—N6—H6B | 107.5 |
| Cu8iii—N2—H2B | 108.8 | C7—O1—Cu1 | 122.0 (4) |
| H2A—N2—H2B | 107.6 | C7—O2—Cu2 | 124.5 (4) |
| C13—C8—C9 | 120.2 (5) | C14—O3—Cu3 | 117.4 (4) |
| C13—C8—C14 | 119.8 (5) | C14—O4—Cu4 | 124.0 (4) |
| C9—C8—C14 | 120.0 (5) | C21—O5—Cu5 | 119.1 (4) |
| C10—C9—C8 | 118.8 (5) | C21—O6—Cu6 | 123.6 (4) |
| C10—C9—H9 | 120.6 | Cu1—Cl1—Cu3ii | 106.78 (7) |
| C8—C9—H9 | 120.6 | Cu2—Cl2—Cu5i | 105.19 (7) |
| C11—C10—C9 | 120.9 (5) | Cu3—Cl3—Cu2iii | 115.93 (8) |
| C11—C10—N3 | 119.3 (5) | Cu4—Cl4—Cu5iv | 124.31 (8) |
| C9—C10—N3 | 119.5 (5) | Cu5—Cl5—Cu1v | 113.33 (7) |
| C12—C11—C10 | 119.5 (5) | Cu6—Cl6—Cu3vi | 118.53 (8) |
| C12—C11—H11 | 120.3 | ||
| C6—C1—C2—C3 | −0.2 (8) | C20—C19—N6—Cu8ix | −88.0 (6) |
| C7—C1—C2—C3 | −179.2 (5) | C18—C19—N6—Cu8ix | 90.3 (6) |
| C1—C2—C3—C4 | −0.1 (8) | O2—C7—O1—Cu1 | 12.7 (7) |
| C1—C2—C3—N1 | 175.3 (5) | C1—C7—O1—Cu1 | −166.7 (4) |
| C2—C3—C4—C5 | 0.8 (8) | Cl1—Cu1—O1—C7 | 93.3 (5) |
| N1—C3—C4—C5 | −174.7 (5) | Cl5i—Cu1—O1—C7 | −114.0 (4) |
| C3—C4—C5—C6 | −1.1 (8) | Cu2—Cu1—O1—C7 | −22.7 (4) |
| C3—C4—C5—N2 | −178.6 (5) | Cu7—Cu1—O1—C7 | 139.2 (5) |
| C4—C5—C6—C1 | 0.7 (8) | O1—C7—O2—Cu2 | 16.1 (8) |
| N2—C5—C6—C1 | 178.2 (5) | C1—C7—O2—Cu2 | −164.5 (4) |
| C2—C1—C6—C5 | 0.0 (8) | Cl2—Cu2—O2—C7 | 89.9 (5) |
| C7—C1—C6—C5 | 179.0 (5) | Cl3ii—Cu2—O2—C7 | −115.3 (5) |
| C2—C1—C7—O2 | 178.4 (5) | Cu1—Cu2—O2—C7 | −24.6 (5) |
| C6—C1—C7—O2 | −0.6 (8) | Cu8—Cu2—O2—C7 | 140.2 (5) |
| C2—C1—C7—O1 | −2.2 (8) | O4—C14—O3—Cu3 | −18.7 (8) |
| C6—C1—C7—O1 | 178.8 (5) | C8—C14—O3—Cu3 | 163.5 (4) |
| C4—C3—N1—Cu9viii | 87.5 (6) | Cl3—Cu3—O3—C14 | −158.1 (4) |
| C2—C3—N1—Cu9viii | −88.0 (6) | Cl1iii—Cu3—O3—C14 | −28.6 (5) |
| C6—C5—N2—Cu8iii | −85.6 (6) | Cl6iv—Cu3—O3—C14 | 98.9 (4) |
| C4—C5—N2—Cu8iii | 92.0 (5) | Cu4—Cu3—O3—C14 | 28.9 (4) |
| C13—C8—C9—C10 | 0.3 (8) | O3—C14—O4—Cu4 | −16.1 (8) |
| C14—C8—C9—C10 | 179.1 (5) | C8—C14—O4—Cu4 | 161.7 (4) |
| C8—C9—C10—C11 | 1.8 (8) | Cl4—Cu4—O4—C14 | −166.7 (4) |
| C8—C9—C10—N3 | −172.2 (5) | Cu3—Cu4—O4—C14 | 29.5 (5) |
| C9—C10—C11—C12 | −2.5 (9) | Cu9—Cu4—O4—C14 | −167.2 (5) |
| N3—C10—C11—C12 | 171.4 (5) | O6—C21—O5—Cu5 | 18.9 (7) |
| C10—C11—C12—C13 | 1.1 (9) | C15—C21—O5—Cu5 | −161.4 (4) |
| C10—C11—C12—N4 | −179.8 (5) | Cl5—Cu5—O5—C21 | 164.7 (4) |
| C11—C12—C13—C8 | 1.1 (9) | Cl2v—Cu5—O5—C21 | 26.4 (5) |
| N4—C12—C13—C8 | −178.1 (5) | Cl4vi—Cu5—O5—C21 | −92.0 (4) |
| C9—C8—C13—C12 | −1.8 (8) | Cu6—Cu5—O5—C21 | −29.9 (4) |
| C14—C8—C13—C12 | 179.5 (5) | O5—C21—O6—Cu6 | 17.4 (7) |
| C13—C8—C14—O4 | 18.2 (8) | C15—C21—O6—Cu6 | −162.3 (4) |
| C9—C8—C14—O4 | −160.5 (5) | Cl6—Cu6—O6—C21 | 169.6 (3) |
| C13—C8—C14—O3 | −163.8 (5) | Cu5—Cu6—O6—C21 | −30.6 (4) |
| C9—C8—C14—O3 | 17.5 (8) | Cu10—Cu6—O6—C21 | 168.2 (5) |
| C11—C10—N3—Cu10vi | −89.8 (6) | O1—Cu1—Cl1—Cu3ii | −90.7 (2) |
| C9—C10—N3—Cu10vi | 84.3 (6) | Cl5i—Cu1—Cl1—Cu3ii | 117.39 (7) |
| C11—C12—N4—Cu7ii | −93.8 (6) | Cu2—Cu1—Cl1—Cu3ii | 15.68 (8) |
| C13—C12—N4—Cu7ii | 85.3 (6) | Cu7—Cu1—Cl1—Cu3ii | −131.23 (6) |
| C20—C15—C16—C17 | 0.5 (8) | O2—Cu2—Cl2—Cu5i | −90.0 (3) |
| C21—C15—C16—C17 | −178.8 (5) | Cl3ii—Cu2—Cl2—Cu5i | 115.39 (7) |
| C15—C16—C17—C18 | −3.0 (9) | Cu1—Cu2—Cl2—Cu5i | 15.51 (8) |
| C15—C16—C17—N5 | 172.9 (5) | Cu8—Cu2—Cl2—Cu5i | −136.75 (6) |
| C16—C17—C18—C19 | 2.7 (8) | O3—Cu3—Cl3—Cu2iii | 118.45 (18) |
| N5—C17—C18—C19 | −173.1 (5) | Cl1iii—Cu3—Cl3—Cu2iii | −13.58 (10) |
| C17—C18—C19—C20 | 0.0 (8) | Cl6iv—Cu3—Cl3—Cu2iii | −141.53 (7) |
| C17—C18—C19—N6 | −178.3 (5) | Cu4—Cu3—Cl3—Cu2iii | −80.2 (2) |
| C18—C19—C20—C15 | −2.4 (8) | O4—Cu4—Cl4—Cu5iv | 95.9 (5) |
| N6—C19—C20—C15 | 175.9 (5) | Cu3—Cu4—Cl4—Cu5iv | −102.07 (9) |
| C16—C15—C20—C19 | 2.1 (8) | Cu9—Cu4—Cl4—Cu5iv | 96.38 (9) |
| C21—C15—C20—C19 | −178.6 (5) | O5—Cu5—Cl5—Cu1v | −120.24 (18) |
| C16—C15—C21—O6 | 161.7 (5) | Cl2v—Cu5—Cl5—Cu1v | 19.58 (10) |
| C20—C15—C21—O6 | −17.6 (7) | Cl4vi—Cu5—Cl5—Cu1v | 140.66 (7) |
| C16—C15—C21—O5 | −18.1 (7) | Cu6—Cu5—Cl5—Cu1v | 92.79 (15) |
| C20—C15—C21—O5 | 162.6 (5) | O6—Cu6—Cl6—Cu3vi | −107.5 (3) |
| C18—C17—N5—Cu9iv | 91.0 (6) | Cu5—Cu6—Cl6—Cu3vi | 97.29 (9) |
| C16—C17—N5—Cu9iv | −84.9 (6) | Cu10—Cu6—Cl6—Cu3vi | −106.27 (8) |
| Symmetry codes: (i) x+1/2, −y−1/2, z−1/2; (ii) −x+1/2, y−1/2, −z+1/2; (iii) −x+1/2, y+1/2, −z+1/2; (iv) −x+1/2, y+1/2, −z+3/2; (v) x−1/2, −y−1/2, z+1/2; (vi) −x+1/2, y−1/2, −z+3/2; (vii) −x+1, −y, −z; (viii) x+1/2, −y+1/2, z−1/2; (ix) x−1/2, −y+1/2, z+1/2; (x) −x, −y, −z+2. |
| D—H···A | D—H | H···A | D···A | D—H···A |
| N1—H1A···Cl1xi | 0.92 | 2.64 | 3.517 (6) | 160 |
| N1—H1B···Cl1vii | 0.92 | 2.74 | 3.657 (6) | 176 |
| N2—H2A···Cl2xi | 0.92 | 2.53 | 3.422 (5) | 164 |
| N2—H2B···Cl3 | 0.92 | 2.54 | 3.440 (5) | 165 |
| N3—H3A···Cl2 | 0.92 | 2.75 | 3.661 (6) | 173 |
| N3—H3B···Cl3xii | 0.92 | 2.57 | 3.471 (5) | 168 |
| N4—H4A···Cl5 | 0.92 | 2.73 | 3.612 (5) | 160 |
| N4—H4B···Cl4xii | 0.92 | 2.49 | 3.354 (5) | 156 |
| N5—H5A···Cl5xi | 0.92 | 2.52 | 3.423 (5) | 168 |
| N5—H5B···Cl4 | 0.92 | 2.65 | 3.557 (6) | 168 |
| N6—H6A···Cl6xi | 0.92 | 2.51 | 3.382 (6) | 159 |
| N6—H6B···Cl6x | 0.92 | 2.73 | 3.634 (6) | 167 |
| Symmetry codes: (vii) −x+1, −y, −z; (x) −x, −y, −z+2; (xi) x, y+1, z; (xii) x, y−1, z. |
Experimental details
| Crystal data | |
| Chemical formula | [Cu3(C7H7CN2O2)Cl2] |
| Mr | 412.67 |
| Crystal system, space group | Monoclinic, P21/n |
| Temperature (K) | 180 |
| a, b, c (Å) | 14.4127 (2), 10.8942 (2), 20.2348 (4) |
| β (°) | 104.8395 (7) |
| V (Å3) | 3071.20 (9) |
| Z | 12 |
| Radiation type | Mo Kα |
| µ (mm−1) | 6.68 |
| Crystal size (mm) | 0.39 × 0.16 × 0.14 |
| Data collection | |
| Diffractometer | Bruker APEXII CCD |
| Absorption correction | Multi-scan (SADABS; Bruker, 2004) |
| Tmin, Tmax | 0.181, 0.455 |
| No. of measured, independent and observed [I > 2σ(I)] reflections | 16907, 5987, 4798 |
| Rint | 0.069 |
| (sin θ/λ)max (Å−1) | 0.617 |
| Refinement | |
| R[F2 > 2σ(F2)], wR(F2), S | 0.069, 0.202, 1.04 |
| No. of reflections | 5987 |
| No. of parameters | 436 |
| H-atom treatment | H-atom parameters constrained |
| w = 1/[σ2(Fo2) + (0.1163P)2 + 23.2868P] where P = (Fo2 + 2Fc2)/3 | |
| Δρmax, Δρmin (e Å−3) | 3.40, −2.71 |
Computer programs: APEX2 (Bruker, 2004), SAINT (Bruker, 2004), SHELXS97 (Sheldrick, 2008), SHELXL2014 (Sheldrick, 2015), ORTEP-3 for Windows (Farrugia, 2012) and ATOMS (Dowty, 2004).
| Cu1—O1 | 1.943 (4) | Cu6—Cl6 | 2.1583 (18) |
| Cu1—Cl1 | 2.1921 (17) | Cu6—Cl4vi | 2.7735 (19) |
| Cu1—Cl5i | 2.3928 (17) | Cu6—Cl2v | 2.7828 (18) |
| Cu2—O2 | 1.929 (5) | Cu7—N4i | 1.929 (5) |
| Cu2—Cl2 | 2.1964 (16) | Cu8—N2ii | 1.938 (5) |
| Cu2—Cl3ii | 2.4246 (18) | Cu8—N6vii | 1.939 (6) |
| Cu3—O3 | 2.033 (5) | Cu8—O3 | 2.697 (4) |
| Cu3—Cl3 | 2.2788 (18) | Cu9—N5vi | 1.953 (5) |
| Cu3—Cl1iii | 2.439 (2) | Cu9—N1viii | 1.957 (5) |
| Cu3—Cl6iv | 2.485 (2) | Cu9—O5 | 2.661 (4) |
| Cu4—O4 | 1.896 (5) | Cu10—N3v | 1.946 (5) |
| Cu4—Cl4 | 2.1415 (17) | Cu10—O6 | 2.798 (4) |
| Cu4—Cl1iii | 2.7981 (19) | C7—O2 | 1.261 (7) |
| Cu5—O5 | 2.024 (4) | C7—O1 | 1.270 (7) |
| Cu5—Cl5 | 2.2672 (17) | C14—O4 | 1.257 (7) |
| Cu5—Cl2v | 2.4009 (18) | C14—O3 | 1.263 (7) |
| Cu5—Cl4vi | 2.5373 (18) | C21—O6 | 1.258 (7) |
| Cu6—O6 | 1.916 (4) | C21—O5 | 1.260 (7) |
| O1—Cu1—Cl1 | 143.31 (15) | O4—Cu4—Cl4 | 161.90 (15) |
| O1—Cu1—Cl5i | 103.78 (15) | O5—Cu5—Cl5 | 124.08 (14) |
| Cl1—Cu1—Cl5i | 108.94 (6) | O5—Cu5—Cl2v | 113.99 (14) |
| O2—Cu2—Cl2 | 147.00 (16) | Cl5—Cu5—Cl2v | 109.72 (7) |
| O2—Cu2—Cl3ii | 103.86 (15) | O5—Cu5—Cl4vi | 91.90 (14) |
| Cl2—Cu2—Cl3ii | 106.14 (6) | Cl5—Cu5—Cl4vi | 99.86 (6) |
| O3—Cu3—Cl3 | 120.86 (15) | Cl2v—Cu5—Cl4vi | 114.90 (7) |
| O3—Cu3—Cl1iii | 113.26 (14) | O6—Cu6—Cl6 | 151.94 (15) |
| Cl3—Cu3—Cl1iii | 107.42 (7) | N4i—Cu7—N4iii | 180.0 |
| O3—Cu3—Cl6iv | 93.82 (16) | N2ii—Cu8—N6vii | 177.8 (2) |
| Cl3—Cu3—Cl6iv | 99.08 (7) | N5vi—Cu9—N1viii | 176.6 (2) |
| Cl1iii—Cu3—Cl6iv | 122.15 (7) | N3v—Cu10—N3iv | 180.0 (3) |
| Symmetry codes: (i) x+1/2, −y−1/2, z−1/2; (ii) −x+1/2, y−1/2, −z+1/2; (iii) −x+1/2, y+1/2, −z+1/2; (iv) −x+1/2, y+1/2, −z+3/2; (v) x−1/2, −y−1/2, z+1/2; (vi) −x+1/2, y−1/2, −z+3/2; (vii) x+1/2, −y+1/2, z−1/2; (viii) x−1/2, −y+1/2, z+1/2. |
| D—H···A | D—H | H···A | D···A | D—H···A |
| N1—H1A···Cl1ix | 0.92 | 2.64 | 3.517 (6) | 160 |
| N1—H1B···Cl1x | 0.92 | 2.74 | 3.657 (6) | 176 |
| N2—H2A···Cl2ix | 0.92 | 2.53 | 3.422 (5) | 164 |
| N2—H2B···Cl3 | 0.92 | 2.54 | 3.440 (5) | 165 |
| N3—H3A···Cl2 | 0.92 | 2.75 | 3.661 (6) | 173 |
| N3—H3B···Cl3xi | 0.92 | 2.57 | 3.471 (5) | 168 |
| N4—H4A···Cl5 | 0.92 | 2.73 | 3.612 (5) | 160 |
| N4—H4B···Cl4xi | 0.92 | 2.49 | 3.354 (5) | 156 |
| N5—H5A···Cl5ix | 0.92 | 2.52 | 3.423 (5) | 168 |
| N5—H5B···Cl4 | 0.92 | 2.65 | 3.557 (6) | 168 |
| N6—H6A···Cl6ix | 0.92 | 2.51 | 3.382 (6) | 159 |
| N6—H6B···Cl6xii | 0.92 | 2.73 | 3.634 (6) | 167 |
| Symmetry codes: (ix) x, y+1, z; (x) −x+1, −y, −z; (xi) x, y−1, z; (xii) −x, −y, −z+2. |

 journal menu
journal menu








![[mu]](/logos/entities/mu_rmgif.gif) -chlorido-(
-chlorido-(![[kappa]](/logos/entities/kappa_rmgif.gif)
![[Figure 1]](http://journals.iucr.org/c/issues/2016/01/00/sk3607/sk3607fig1thm.gif)
![[Figure 2]](http://journals.iucr.org/c/issues/2016/01/00/sk3607/sk3607fig2thm.gif)
![[Figure 3]](http://journals.iucr.org/c/issues/2016/01/00/sk3607/sk3607fig3thm.gif)
![[Figure 4]](http://journals.iucr.org/c/issues/2016/01/00/sk3607/sk3607fig4thm.gif)
![[Figure 5]](http://journals.iucr.org/c/issues/2016/01/00/sk3607/sk3607fig5thm.gif)
![[Figure 6]](http://journals.iucr.org/c/issues/2016/01/00/sk3607/sk3607fig6thm.gif)



