The structure of tetragonal (P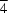 21m) ammonium chlorite, NH4ClO2, has been redetermined by single-crystal X-ray analysis with higher precision. The NH4+ cation is at a site of
21m) ammonium chlorite, NH4ClO2, has been redetermined by single-crystal X-ray analysis with higher precision. The NH4+ cation is at a site of  symmetry, while the unique Cl and O atoms are at sites with symmetries mm and m, respectively. The structure consists of corrugated layers of NH4+ cations coordinated by ClO2- anions. The H atoms of the ammonium cation hydrogen bond to four chlorite O atoms. The resultant coordination is almost ideal tetrahedral.
symmetry, while the unique Cl and O atoms are at sites with symmetries mm and m, respectively. The structure consists of corrugated layers of NH4+ cations coordinated by ClO2- anions. The H atoms of the ammonium cation hydrogen bond to four chlorite O atoms. The resultant coordination is almost ideal tetrahedral.
The structure of calcium chlorite, Ca(ClO2)2, has been refined from X-ray powder diffraction data using the Rietveld method. The compound crystallizes in the orthorhombic space group Ccca (Ccce), with Z = 4. The structure is based on separate layers parallel to the ac plane, consisting of calcium cations that are coordinated by chlorite anions; the O atoms form almost ideal square antiprisms. Within the layers, each anion bridges four metal cations. The Ca atoms are located on special positions of 222 symmetry, the Cl atoms lie on twofold axes and the O atoms are in general positions. The compound is isostructural with strontium chlorite, Sr(ClO2)2, and lead chlorite, Pb(ClO2)2.
The structure of strontium chlorite, Sr(ClO2)2, has been refined from X-ray powder diffraction data using the Rietveld method. The compound crystallizes in the orthorhombic space group Ccca (Ccce), with Z = 4. The structure is based on separate layers parallel to the ac plane, consisting of strontium cations that are coordinated by chlorite anions; the O atoms form almost ideal square antiprisms. Within the layers, each anion bridges four metal cations. The Sr atoms are located on special positions of 222 symmetry, the Cl atoms lie on twofold axes and the O atoms are in general positions. The compound is isostructural with calcium chlorite, Ca(ClO2)2, and lead chlorite, Pb(ClO2)2.

 journal menu
journal menu












