![[mu]](/logos/entities/mu_rmgif.gif) -oxalato-dibismuth(III) dihydrate, {[Bi2(C2O4)3(H2O)4]·2H2O}n) and dibismuth(III) trioxalate octahydrate (tetraaquatri-
-oxalato-dibismuth(III) dihydrate, {[Bi2(C2O4)3(H2O)4]·2H2O}n) and dibismuth(III) trioxalate octahydrate (tetraaquatri-![[mu]](/logos/entities/mu_rmgif.gif) -oxalato-dibismuth(III) tetrahydrate {[Bi2(C2O4)3(H2O)4]·4H2O}n) are characterized by a three-dimensional network of Bi atoms connected by tetradentate oxalate groups. All ligand and `free' water molecules are located in channels and voids. The mean Bi-O bond lengths are
-oxalato-dibismuth(III) tetrahydrate {[Bi2(C2O4)3(H2O)4]·4H2O}n) are characterized by a three-dimensional network of Bi atoms connected by tetradentate oxalate groups. All ligand and `free' water molecules are located in channels and voids. The mean Bi-O bond lengths are 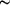 2.51 Å. The lone electron pairs on all Bi3+ cations are stereochemically inactive.
2.51 Å. The lone electron pairs on all Bi3+ cations are stereochemically inactive.Supporting information
Crystallographic Information File (CIF) https://doi.org/10.1107/S0108270103023618/ta1420sup1.cif | |
Structure factor file (CIF format) https://doi.org/10.1107/S0108270103023618/ta1420Isup2.hkl | |
Structure factor file (CIF format) https://doi.org/10.1107/S0108270103023618/ta1420IIsup3.hkl |
CCDC references: 229063; 229064
Both title compounds crystallized at room temperature from the same aqueous solution of Bi(NO3)3·5H2O and oxalic acid dihydrate. Bi2(C2O4)3·6H2O formed small thick-tabular slightly rounded colorless crystals. Bi2(C2O4)3·8H2O crystallized as colorless prisms arranged in sprays.
The highest peak in Bi2(C2O4)3·6H2O, 1.74 e/Å3, is 1.43 Å from the O1-atom site; the deepest hole, −1.85 e/Å3, is 0.88 Å from the Bi-atom site. The highest peak in Bi2(C2O4)3·8H2O, 1.73 e/Å3, is 1.48 Å from the O2-atom site; the deepest hole, −1.87 e/Å3, is 0.79 Å from the Bi1-atom site.
For both compounds, data collection: COLLECT (Nonius, 2002); cell refinement: HKL SCALEPACK (Otwinowski & Minor, 1997); data reduction: HKL DENZO (Otwinowski & Minor, 1997) and SCALEPACK; program(s) used to solve structure: SHELXS97 (Sheldrick, 1997); program(s) used to refine structure: SHELXL97 (Sheldrick, 1997); molecular graphics: ATOMS (Shape Software, 1999).
| [Bi2(C2O4)3(H2O)4]·2H2O | F(000) = 716 |
| Mr = 790.12 | Dx = 3.244 Mg m−3 |
| Monoclinic, P21/c | Mo Kα radiation, λ = 0.71073 Å |
| Hall symbol: -P 2ybc | Cell parameters from 3097 reflections |
| a = 9.776 (2) Å | θ = 2.0–32.6° |
| b = 8.211 (2) Å | µ = 21.82 mm−1 |
| c = 10.224 (2) Å | T = 293 K |
| β = 99.75 (3)° | Fragment, colorless |
| V = 808.8 (3) Å3 | 0.09 × 0.07 × 0.04 mm |
| Z = 2 |
| Nonius KappaCCD diffractometer | 2924 independent reflections |
| Radiation source: fine-focus sealed tube | 2670 reflections with I > 2σ(I) |
| Graphite monochromator | Rint = 0.013 |
| ϕ and ω scans | θmax = 32.6°, θmin = 3.3° |
| Absorption correction: multi-scan (HKL SCALEPACK; Otwinowski & Minor, 1997) | h = −14→14 |
| Tmin = 0.244, Tmax = 0.476 | k = −12→12 |
| 5650 measured reflections | l = −15→15 |
| Refinement on F2 | Secondary atom site location: difference Fourier map |
| Least-squares matrix: full | H-atom parameters not refined |
| R[F2 > 2σ(F2)] = 0.019 | w = 1/[σ2(Fo2) + (0.021P)2 + 1.950P] where P = (Fo2 + 2Fc2)/3 |
| wR(F2) = 0.048 | (Δ/σ)max = 0.001 |
| S = 1.06 | Δρmax = 1.74 e Å−3 |
| 2924 reflections | Δρmin = −1.85 e Å−3 |
| 119 parameters | Extinction correction: SHELXL97, Fc*=kFc[1+0.001xFc2λ3/sin(2θ)]-1/4 |
| 0 restraints | Extinction coefficient: 0.00226 (18) |
| Primary atom site location: structure-invariant direct methods |
| [Bi2(C2O4)3(H2O)4]·2H2O | V = 808.8 (3) Å3 |
| Mr = 790.12 | Z = 2 |
| Monoclinic, P21/c | Mo Kα radiation |
| a = 9.776 (2) Å | µ = 21.82 mm−1 |
| b = 8.211 (2) Å | T = 293 K |
| c = 10.224 (2) Å | 0.09 × 0.07 × 0.04 mm |
| β = 99.75 (3)° |
| Nonius KappaCCD diffractometer | 2924 independent reflections |
| Absorption correction: multi-scan (HKL SCALEPACK; Otwinowski & Minor, 1997) | 2670 reflections with I > 2σ(I) |
| Tmin = 0.244, Tmax = 0.476 | Rint = 0.013 |
| 5650 measured reflections |
| R[F2 > 2σ(F2)] = 0.019 | 0 restraints |
| wR(F2) = 0.048 | H-atom parameters not refined |
| S = 1.06 | Δρmax = 1.74 e Å−3 |
| 2924 reflections | Δρmin = −1.85 e Å−3 |
| 119 parameters |
Geometry. All e.s.d.'s (except the e.s.d. in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell e.s.d.'s are taken into account individually in the estimation of e.s.d.'s in distances, angles and torsion angles; correlations between e.s.d.'s in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell e.s.d.'s is used for estimating e.s.d.'s involving l.s. planes. |
Refinement. Refinement of F2 against ALL reflections. The weighted R-factor wR and goodness of fit S are based on F2, conventional R-factors R are based on F, with F set to zero for negative F2. The threshold expression of F2 > σ(F2) is used only for calculating R-factors(gt) etc. and is not relevant to the choice of reflections for refinement. R-factors based on F2 are statistically about twice as large as those based on F, and R- factors based on ALL data will be even larger. |
| x | y | z | Uiso*/Ueq | ||
| Bi | 0.185412 (10) | 0.092537 (13) | 0.927452 (10) | 0.01836 (5) | |
| C1 | 0.4959 (3) | −0.0176 (4) | 1.0739 (3) | 0.0229 (6) | |
| C2 | 0.0833 (3) | 0.3989 (3) | 0.7330 (3) | 0.0176 (5) | |
| C3 | −0.0251 (3) | 0.4057 (3) | 0.8276 (3) | 0.0192 (5) | |
| O1 | 0.3806 (2) | −0.0005 (4) | 1.1079 (2) | 0.0359 (7) | |
| O2 | 0.6046 (2) | −0.0629 (4) | 1.1482 (2) | 0.0299 (5) | |
| O3 | 0.1858 (2) | 0.3081 (3) | 0.7640 (2) | 0.0255 (5) | |
| O4 | 0.0599 (2) | 0.4859 (3) | 0.6305 (2) | 0.0235 (4) | |
| O5 | −0.1287 (3) | 0.4958 (3) | 0.7945 (2) | 0.0280 (5) | |
| O6 | −0.0003 (2) | 0.3210 (3) | 0.9299 (2) | 0.0253 (4) | |
| OW1 | 0.1861 (3) | −0.2057 (4) | 0.9273 (3) | 0.0411 (7) | |
| OW2 | 0.3158 (3) | 0.3531 (4) | 1.0419 (3) | 0.0372 (6) | |
| OW3 | 0.6091 (3) | 0.1011 (3) | 0.6288 (3) | 0.0366 (6) |
| U11 | U22 | U33 | U12 | U13 | U23 | |
| Bi | 0.01254 (6) | 0.02490 (7) | 0.01804 (6) | 0.00189 (3) | 0.00372 (4) | 0.00248 (4) |
| C1 | 0.0151 (12) | 0.0358 (17) | 0.0185 (12) | 0.0049 (11) | 0.0052 (10) | 0.0051 (11) |
| C2 | 0.0159 (12) | 0.0204 (13) | 0.0168 (12) | −0.0030 (9) | 0.0037 (10) | 0.0003 (9) |
| C3 | 0.0188 (13) | 0.0211 (13) | 0.0180 (12) | 0.0014 (10) | 0.0040 (10) | −0.0018 (9) |
| O1 | 0.0180 (11) | 0.068 (2) | 0.0236 (11) | 0.0165 (11) | 0.0089 (9) | 0.0149 (12) |
| O2 | 0.0152 (10) | 0.0538 (16) | 0.0208 (10) | 0.0091 (10) | 0.0039 (8) | 0.0110 (10) |
| O3 | 0.0216 (10) | 0.0296 (12) | 0.0266 (11) | 0.0061 (9) | 0.0076 (8) | 0.0084 (9) |
| O4 | 0.0176 (10) | 0.0313 (12) | 0.0211 (10) | 0.0012 (8) | 0.0021 (8) | 0.0082 (8) |
| O5 | 0.0279 (11) | 0.0352 (13) | 0.0217 (10) | 0.0121 (10) | 0.0061 (8) | 0.0028 (9) |
| O6 | 0.0254 (11) | 0.0282 (11) | 0.0240 (10) | 0.0057 (9) | 0.0091 (8) | 0.0062 (9) |
| OW1 | 0.0400 (16) | 0.0359 (16) | 0.0546 (19) | 0.0098 (11) | 0.0286 (14) | 0.0124 (12) |
| OW2 | 0.0406 (16) | 0.0449 (16) | 0.0251 (12) | −0.0123 (12) | 0.0025 (10) | −0.0054 (12) |
| OW3 | 0.0361 (15) | 0.0430 (16) | 0.0316 (14) | −0.0053 (11) | 0.0083 (11) | 0.0053 (11) |
| Bi—O2i | 2.326 (2) | C1—O2 | 1.253 (4) |
| Bi—O5ii | 2.380 (2) | C1—C1i | 1.553 (6) |
| Bi—O3 | 2.435 (2) | C2—O3 | 1.246 (4) |
| Bi—OW1 | 2.449 (3) | C2—O4 | 1.257 (4) |
| Bi—O4ii | 2.527 (2) | C2—C3 | 1.553 (4) |
| Bi—O1 | 2.537 (2) | C3—O6 | 1.245 (4) |
| Bi—O6 | 2.614 (2) | C3—O5 | 1.254 (4) |
| Bi—OW2 | 2.658 (3) | OW3—Biiv | 4.587 (3) |
| Bi—O4iii | 2.663 (2) | OW3—Biv | 4.711 (3) |
| C1—O1 | 1.243 (4) | ||
| O2i—Bi—O5ii | 73.97 (9) | O3—Bi—OW2 | 70.16 (9) |
| O2i—Bi—O3 | 75.15 (8) | OW1—Bi—OW2 | 143.50 (10) |
| O5ii—Bi—O3 | 67.38 (9) | O4ii—Bi—OW2 | 138.35 (8) |
| O2i—Bi—OW1 | 83.82 (9) | O1—Bi—OW2 | 71.14 (10) |
| O5ii—Bi—OW1 | 70.48 (9) | O6—Bi—OW2 | 72.55 (9) |
| O3—Bi—OW1 | 136.58 (9) | O2i—Bi—O4iii | 141.55 (8) |
| O2i—Bi—O4ii | 137.87 (9) | O5ii—Bi—O4iii | 126.81 (7) |
| O5ii—Bi—O4ii | 66.57 (8) | O3—Bi—O4iii | 139.93 (7) |
| O3—Bi—O4ii | 101.71 (8) | OW1—Bi—O4iii | 76.14 (8) |
| OW1—Bi—O4ii | 69.87 (9) | O4ii—Bi—O4iii | 63.56 (8) |
| O2i—Bi—O1 | 67.22 (8) | O1—Bi—O4iii | 75.49 (7) |
| O5ii—Bi—O1 | 128.17 (9) | O6—Bi—O4iii | 75.73 (7) |
| O3—Bi—O1 | 129.02 (9) | OW2—Bi—O4iii | 95.81 (8) |
| OW1—Bi—O1 | 72.39 (11) | O1—C1—O2 | 125.4 (3) |
| O4ii—Bi—O1 | 129.27 (9) | O1—C1—C1i | 116.7 (3) |
| O2i—Bi—O6 | 137.05 (8) | O2—C1—C1i | 117.9 (3) |
| O5ii—Bi—O6 | 101.46 (9) | O3—C2—O4 | 125.3 (3) |
| O3—Bi—O6 | 64.31 (7) | O3—C2—C3 | 117.8 (3) |
| OW1—Bi—O6 | 136.04 (8) | O4—C2—C3 | 116.9 (3) |
| O4ii—Bi—O6 | 67.54 (8) | O6—C3—O5 | 126.1 (3) |
| O1—Bi—O6 | 130.34 (8) | O6—C3—C2 | 116.6 (3) |
| O2i—Bi—OW2 | 81.17 (10) | O5—C3—C2 | 117.3 (3) |
| O5ii—Bi—OW2 | 134.86 (8) | ||
| O3—C2—C3—O6 | 1.2 (4) | O3—C2—C3—O5 | −179.1 (3) |
| O4—C2—C3—O6 | −179.0 (3) | O4—C2—C3—O5 | 0.7 (4) |
| Symmetry codes: (i) −x+1, −y, −z+2; (ii) −x, y−1/2, −z+3/2; (iii) x, −y+1/2, z+1/2; (iv) −x+1, y+1/2, −z+3/2; (v) −x+1, y−1/2, −z+3/2. |
| [Bi2(C2O4)3(H2O)4]·4H2O | Z = 2 |
| Mr = 826.15 | F(000) = 756 |
| Triclinic, P1 | Dx = 3.115 Mg m−3 |
| Hall symbol: -P 1 | Mo Kα radiation, λ = 0.71073 Å |
| a = 9.194 (2) Å | Cell parameters from 5313 reflections |
| b = 9.458 (2) Å | θ = 2.0–32.6° |
| c = 11.176 (2) Å | µ = 20.06 mm−1 |
| α = 101.15 (3)° | T = 293 K |
| β = 101.76 (3)° | Prism, colorless |
| γ = 106.17 (3)° | 0.18 × 0.03 × 0.03 mm |
| V = 880.9 (4) Å3 |
| Nonius KappaCCD diffractometer | 6122 independent reflections |
| Radiation source: fine-focus sealed tube | 4981 reflections with I > 2σ(I) |
| Graphite monochromator | Rint = 0.029 |
| ϕ and ω scans | θmax = 32.6°, θmin = 2.4° |
| Absorption correction: multi-scan (HKL SCALEPACK; Otwinowski & Minor, 1997) | h = −13→13 |
| Tmin = 0.123, Tmax = 0.585 | k = −14→14 |
| 10808 measured reflections | l = −16→16 |
| Refinement on F2 | Secondary atom site location: difference Fourier map |
| Least-squares matrix: full | H-atom parameters not refined |
| R[F2 > 2σ(F2)] = 0.028 | w = 1/[σ2(Fo2) + (0.029P)2 + 0.6P] where P = (Fo2 + 2Fc2)/3 |
| wR(F2) = 0.067 | (Δ/σ)max = 0.001 |
| S = 1.01 | Δρmax = 1.73 e Å−3 |
| 6122 reflections | Δρmin = −1.87 e Å−3 |
| 264 parameters | Extinction correction: SHELXL97, Fc*=kFc[1+0.001xFc2λ3/sin(2θ)]-1/4 |
| 0 restraints | Extinction coefficient: 0.0050 (2) |
| Primary atom site location: structure-invariant direct methods |
| [Bi2(C2O4)3(H2O)4]·4H2O | γ = 106.17 (3)° |
| Mr = 826.15 | V = 880.9 (4) Å3 |
| Triclinic, P1 | Z = 2 |
| a = 9.194 (2) Å | Mo Kα radiation |
| b = 9.458 (2) Å | µ = 20.06 mm−1 |
| c = 11.176 (2) Å | T = 293 K |
| α = 101.15 (3)° | 0.18 × 0.03 × 0.03 mm |
| β = 101.76 (3)° |
| Nonius KappaCCD diffractometer | 6122 independent reflections |
| Absorption correction: multi-scan (HKL SCALEPACK; Otwinowski & Minor, 1997) | 4981 reflections with I > 2σ(I) |
| Tmin = 0.123, Tmax = 0.585 | Rint = 0.029 |
| 10808 measured reflections |
| R[F2 > 2σ(F2)] = 0.028 | 0 restraints |
| wR(F2) = 0.067 | H-atom parameters not refined |
| S = 1.01 | Δρmax = 1.73 e Å−3 |
| 6122 reflections | Δρmin = −1.87 e Å−3 |
| 264 parameters |
Geometry. All e.s.d.'s (except the e.s.d. in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell e.s.d.'s are taken into account individually in the estimation of e.s.d.'s in distances, angles and torsion angles; correlations between e.s.d.'s in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell e.s.d.'s is used for estimating e.s.d.'s involving l.s. planes. |
Refinement. Refinement of F2 against ALL reflections. The weighted R-factor wR and goodness of fit S are based on F2, conventional R-factors R are based on F, with F set to zero for negative F2. The threshold expression of F2 > σ(F2) is used only for calculating R-factors(gt) etc. and is not relevant to the choice of reflections for refinement. R-factors based on F2 are statistically about twice as large as those based on F, and R- factors based on ALL data will be even larger. |
| x | y | z | Uiso*/Ueq | Occ. (<1) | |
| Bi1 | 0.238507 (17) | 0.658354 (17) | 0.126577 (14) | 0.01630 (5) | |
| Bi2 | 0.080104 (17) | 0.885970 (17) | −0.356471 (14) | 0.01648 (5) | |
| C1 | 0.2371 (5) | 0.7895 (5) | −0.1128 (4) | 0.0172 (8) | |
| C2 | 0.0704 (5) | 0.6693 (5) | −0.1595 (4) | 0.0183 (8) | |
| C3 | −0.0776 (5) | 1.0839 (5) | −0.1980 (4) | 0.0181 (8) | |
| C4 | −0.0835 (5) | 1.1511 (5) | −0.3150 (4) | 0.0169 (8) | |
| C5 | 0.6123 (5) | 0.8086 (5) | 0.2301 (4) | 0.0182 (8) | |
| C6 | 0.5465 (5) | 0.9224 (5) | 0.3020 (4) | 0.0191 (8) | |
| O1 | 0.2684 (4) | 0.8872 (4) | −0.1726 (3) | 0.0279 (7) | |
| O2 | 0.3331 (4) | 0.7833 (4) | −0.0169 (3) | 0.0238 (7) | |
| O3 | 0.0332 (4) | 0.5809 (4) | −0.0925 (3) | 0.0226 (6) | |
| O4 | −0.0146 (3) | 0.6709 (4) | −0.2620 (3) | 0.0238 (7) | |
| O5 | −0.1094 (4) | 1.1524 (4) | −0.1050 (3) | 0.0285 (8) | |
| O6 | −0.0408 (4) | 0.9656 (4) | −0.2023 (3) | 0.0266 (7) | |
| O7 | −0.1115 (4) | 1.2743 (4) | −0.3041 (3) | 0.0265 (7) | |
| O8 | −0.0594 (4) | 1.0767 (4) | −0.4096 (3) | 0.0212 (6) | |
| O9 | 0.7583 (4) | 0.8484 (4) | 0.2476 (3) | 0.0260 (7) | |
| O10 | 0.5146 (4) | 0.6832 (4) | 0.1601 (3) | 0.0251 (7) | |
| O11 | 0.6417 (4) | 1.0370 (4) | 0.3831 (3) | 0.0265 (7) | |
| O12 | 0.3984 (4) | 0.8859 (4) | 0.2737 (3) | 0.0265 (7) | |
| OW1 | −0.1963 (4) | 0.7271 (4) | −0.4784 (4) | 0.0359 (9) | |
| OW2 | 0.2659 (5) | 0.4210 (5) | −0.0195 (4) | 0.0450 (10) | |
| OW3 | 0.3098 (4) | 0.5299 (4) | 0.3032 (3) | 0.0264 (7) | |
| OW4 | 0.1190 (5) | 0.6471 (4) | −0.4747 (4) | 0.0379 (9) | |
| OW5 | 0.4763 (5) | 0.3419 (5) | 0.2638 (4) | 0.0483 (11) | |
| OW6 | 0.4911 (4) | 0.2400 (4) | 0.4788 (4) | 0.0380 (9) | |
| OW7 | 0.7728 (5) | 0.5271 (5) | 0.2982 (4) | 0.0514 (11) | |
| OW8A | 0.330 (6) | 0.142 (2) | 0.054 (2) | 0.043 (8) | 0.31 (6) |
| OW8B | 0.249 (3) | 0.1533 (13) | 0.0451 (8) | 0.056 (5) | 0.69 (6) |
| U11 | U22 | U33 | U12 | U13 | U23 | |
| Bi1 | 0.01572 (8) | 0.01679 (8) | 0.01499 (8) | 0.00409 (6) | 0.00280 (5) | 0.00439 (6) |
| Bi2 | 0.01698 (8) | 0.01621 (8) | 0.01541 (8) | 0.00434 (6) | 0.00402 (6) | 0.00442 (6) |
| C1 | 0.0167 (19) | 0.019 (2) | 0.017 (2) | 0.0070 (16) | 0.0049 (15) | 0.0043 (16) |
| C2 | 0.019 (2) | 0.021 (2) | 0.016 (2) | 0.0068 (16) | 0.0055 (15) | 0.0061 (16) |
| C3 | 0.0163 (19) | 0.021 (2) | 0.018 (2) | 0.0068 (16) | 0.0051 (15) | 0.0054 (16) |
| C4 | 0.0134 (18) | 0.020 (2) | 0.0163 (19) | 0.0045 (15) | 0.0061 (14) | 0.0023 (15) |
| C5 | 0.0165 (19) | 0.019 (2) | 0.022 (2) | 0.0067 (16) | 0.0047 (15) | 0.0108 (16) |
| C6 | 0.019 (2) | 0.020 (2) | 0.019 (2) | 0.0074 (16) | 0.0039 (16) | 0.0066 (16) |
| O1 | 0.0235 (16) | 0.0297 (18) | 0.0264 (18) | −0.0007 (13) | −0.0002 (13) | 0.0194 (15) |
| O2 | 0.0206 (15) | 0.0284 (17) | 0.0209 (16) | 0.0040 (13) | 0.0029 (12) | 0.0121 (13) |
| O3 | 0.0241 (16) | 0.0226 (16) | 0.0174 (15) | 0.0008 (12) | 0.0025 (12) | 0.0105 (13) |
| O4 | 0.0196 (15) | 0.0272 (17) | 0.0198 (16) | 0.0022 (13) | −0.0005 (12) | 0.0099 (13) |
| O5 | 0.040 (2) | 0.038 (2) | 0.0197 (17) | 0.0265 (16) | 0.0115 (14) | 0.0106 (15) |
| O6 | 0.0369 (19) | 0.0305 (19) | 0.0241 (17) | 0.0192 (15) | 0.0159 (14) | 0.0140 (14) |
| O7 | 0.0369 (19) | 0.0240 (17) | 0.0287 (19) | 0.0151 (15) | 0.0204 (15) | 0.0102 (14) |
| O8 | 0.0245 (16) | 0.0278 (17) | 0.0164 (15) | 0.0146 (13) | 0.0072 (12) | 0.0070 (13) |
| O9 | 0.0132 (14) | 0.0222 (16) | 0.040 (2) | 0.0050 (12) | 0.0055 (13) | 0.0048 (14) |
| O10 | 0.0172 (15) | 0.0202 (16) | 0.0332 (19) | 0.0046 (12) | 0.0042 (13) | 0.0019 (14) |
| O11 | 0.0209 (16) | 0.0271 (17) | 0.0218 (17) | 0.0007 (13) | 0.0036 (12) | −0.0028 (13) |
| O12 | 0.0182 (15) | 0.0249 (17) | 0.0299 (18) | 0.0045 (13) | 0.0054 (13) | −0.0021 (14) |
| OW1 | 0.0193 (17) | 0.039 (2) | 0.038 (2) | 0.0029 (15) | −0.0007 (14) | 0.0039 (17) |
| OW2 | 0.051 (3) | 0.036 (2) | 0.045 (3) | 0.0211 (19) | 0.0110 (19) | −0.0037 (18) |
| OW3 | 0.0251 (17) | 0.0228 (17) | 0.0296 (19) | 0.0063 (13) | 0.0027 (13) | 0.0107 (14) |
| OW4 | 0.060 (3) | 0.036 (2) | 0.029 (2) | 0.0250 (19) | 0.0200 (18) | 0.0114 (16) |
| OW5 | 0.048 (3) | 0.043 (3) | 0.055 (3) | 0.015 (2) | 0.012 (2) | 0.017 (2) |
| OW6 | 0.034 (2) | 0.040 (2) | 0.037 (2) | 0.0162 (17) | 0.0053 (16) | 0.0043 (17) |
| OW7 | 0.044 (2) | 0.057 (3) | 0.049 (3) | 0.017 (2) | 0.010 (2) | 0.006 (2) |
| OW8A | 0.06 (2) | 0.039 (8) | 0.048 (9) | 0.028 (9) | 0.025 (9) | 0.021 (7) |
| OW8B | 0.071 (12) | 0.040 (4) | 0.045 (4) | 0.007 (5) | 0.008 (4) | 0.009 (3) |
| Bi1—O12 | 2.320 (3) | C2—O3 | 1.251 (5) |
| Bi1—O2 | 2.335 (3) | C2—O4 | 1.253 (5) |
| Bi1—O10 | 2.426 (3) | C3—O5 | 1.247 (5) |
| Bi1—O5i | 2.430 (3) | C3—O6 | 1.251 (5) |
| Bi1—O7i | 2.564 (3) | C3—C4 | 1.557 (6) |
| Bi1—OW3 | 2.577 (3) | C4—O8 | 1.248 (5) |
| Bi1—O3 | 2.597 (3) | C4—O7 | 1.251 (5) |
| Bi1—OW2 | 2.614 (4) | C5—O9 | 1.250 (5) |
| Bi1—O3ii | 2.763 (3) | C5—O10 | 1.260 (5) |
| Bi2—O6 | 2.349 (3) | C5—C6 | 1.548 (6) |
| Bi2—O1 | 2.398 (3) | C6—O11 | 1.237 (5) |
| Bi2—O9iii | 2.450 (3) | C6—O12 | 1.262 (5) |
| Bi2—O4 | 2.496 (3) | OW6—Bi1v | 4.372 (4) |
| Bi2—OW1 | 2.498 (4) | OW6—Bi2vi | 4.797 (4) |
| Bi2—OW4 | 2.543 (4) | OW7—Bi2vii | 4.421 (5) |
| Bi2—O11iii | 2.553 (3) | OW7—Bi2vi | 4.592 (5) |
| Bi2—O8 | 2.577 (3) | OW7—Bi1vi | 4.701 (5) |
| Bi2—O8iv | 2.681 (3) | OW8A—OW8B | 0.77 (2) |
| C1—O1 | 1.246 (5) | OW8A—Bi2viii | 4.52 (3) |
| C1—O2 | 1.265 (5) | OW8A—Bi1viii | 4.676 (18) |
| C1—C2 | 1.545 (6) | OW8B—Bi2viii | 4.420 (9) |
| O12—Bi1—O2 | 82.98 (12) | O4—Bi2—OW1 | 70.30 (12) |
| O12—Bi1—O10 | 68.74 (11) | O6—Bi2—OW4 | 140.83 (12) |
| O2—Bi1—O10 | 72.08 (11) | O1—Bi2—OW4 | 88.27 (13) |
| O12—Bi1—O5i | 72.54 (12) | O9iii—Bi2—OW4 | 137.34 (12) |
| O2—Bi1—O5i | 72.66 (11) | O4—Bi2—OW4 | 69.98 (12) |
| O10—Bi1—O5i | 129.82 (12) | OW1—Bi2—OW4 | 78.44 (13) |
| O12—Bi1—O7i | 69.07 (11) | O6—Bi2—O11iii | 135.59 (12) |
| O2—Bi1—O7i | 134.58 (11) | O1—Bi2—O11iii | 68.82 (11) |
| O10—Bi1—O7i | 124.44 (12) | O9iii—Bi2—O11iii | 65.35 (11) |
| O5i—Bi1—O7i | 65.27 (11) | O4—Bi2—O11iii | 120.84 (11) |
| O12—Bi1—OW3 | 85.67 (12) | OW1—Bi2—O11iii | 140.77 (11) |
| O2—Bi1—OW3 | 144.74 (11) | OW4—Bi2—O11iii | 72.37 (13) |
| O10—Bi1—OW3 | 72.69 (11) | O6—Bi2—O8 | 66.73 (10) |
| O5i—Bi1—OW3 | 134.45 (11) | O1—Bi2—O8 | 133.50 (11) |
| O7i—Bi1—OW3 | 69.70 (11) | O9iii—Bi2—O8 | 67.51 (11) |
| O12—Bi1—O3 | 135.90 (12) | O4—Bi2—O8 | 127.31 (10) |
| O2—Bi1—O3 | 67.04 (10) | OW1—Bi2—O8 | 74.36 (12) |
| O10—Bi1—O3 | 125.57 (11) | OW4—Bi2—O8 | 137.56 (11) |
| O5i—Bi1—O3 | 68.16 (11) | O11iii—Bi2—O8 | 111.35 (11) |
| O7i—Bi1—O3 | 109.83 (11) | O6—Bi2—O8iv | 131.09 (10) |
| OW3—Bi1—O3 | 136.96 (10) | O1—Bi2—O8iv | 141.05 (10) |
| O12—Bi1—OW2 | 139.05 (12) | O9iii—Bi2—O8iv | 94.92 (11) |
| O2—Bi1—OW2 | 83.47 (13) | O4—Bi2—O8iv | 136.57 (10) |
| O10—Bi1—OW2 | 70.34 (13) | OW1—Bi2—O8iv | 76.06 (12) |
| O5i—Bi1—OW2 | 137.89 (13) | OW4—Bi2—O8iv | 77.12 (11) |
| O7i—Bi1—OW2 | 140.43 (13) | O11iii—Bi2—O8iv | 72.35 (11) |
| OW3—Bi1—OW2 | 83.57 (13) | O8—Bi2—O8iv | 65.17 (11) |
| O3—Bi1—OW2 | 70.74 (13) | O1—C1—O2 | 124.0 (4) |
| O12—Bi1—O3ii | 140.09 (11) | O1—C1—C2 | 117.5 (4) |
| O2—Bi1—O3ii | 130.95 (10) | O2—C1—C2 | 118.5 (4) |
| O10—Bi1—O3ii | 134.30 (10) | O3—C2—O4 | 126.5 (4) |
| O5i—Bi1—O3ii | 95.85 (11) | O3—C2—C1 | 117.6 (4) |
| O7i—Bi1—O3ii | 71.41 (11) | O4—C2—C1 | 115.9 (4) |
| OW3—Bi1—O3ii | 75.71 (10) | O5—C3—O6 | 123.9 (4) |
| O3—Bi1—O3ii | 64.51 (11) | O5—C3—C4 | 117.6 (4) |
| OW2—Bi1—O3ii | 74.10 (12) | O6—C3—C4 | 118.5 (4) |
| O6—Bi2—O1 | 81.65 (12) | O8—C4—O7 | 126.8 (4) |
| O6—Bi2—O9iii | 74.46 (12) | O8—C4—C3 | 116.7 (4) |
| O1—Bi2—O9iii | 71.81 (12) | O7—C4—C3 | 116.6 (4) |
| O6—Bi2—O4 | 71.33 (11) | O9—C5—O10 | 125.6 (4) |
| O1—Bi2—O4 | 66.14 (11) | O9—C5—C6 | 117.1 (4) |
| O9iii—Bi2—O4 | 128.50 (11) | O10—C5—C6 | 117.3 (4) |
| O6—Bi2—OW1 | 83.20 (13) | O11—C6—O12 | 126.2 (4) |
| O1—Bi2—OW1 | 136.42 (12) | O11—C6—C5 | 117.9 (4) |
| O9iii—Bi2—OW1 | 140.93 (12) | O12—C6—C5 | 115.9 (4) |
| O1—C1—C2—O3 | −172.9 (4) | O5—C3—C4—O7 | 3.8 (6) |
| O2—C1—C2—O3 | 7.1 (6) | O6—C3—C4—O7 | −175.9 (4) |
| O1—C1—C2—O4 | 6.9 (6) | O9—C5—C6—O11 | −8.3 (6) |
| O2—C1—C2—O4 | −173.1 (4) | O10—C5—C6—O11 | 170.9 (4) |
| O5—C3—C4—O8 | −176.4 (4) | O9—C5—C6—O12 | 173.6 (4) |
| O6—C3—C4—O8 | 3.9 (6) | O10—C5—C6—O12 | −7.2 (6) |
| Symmetry codes: (i) −x, −y+2, −z; (ii) −x, −y+1, −z; (iii) −x+1, −y+2, −z; (iv) −x, −y+2, −z−1; (v) −x+1, −y+1, −z+1; (vi) −x+1, −y+1, −z; (vii) x+1, y, z+1; (viii) x, y−1, z. |
Experimental details
| (I) | (II) | |
| Crystal data | ||
| Chemical formula | [Bi2(C2O4)3(H2O)4]·2H2O | [Bi2(C2O4)3(H2O)4]·4H2O |
| Mr | 790.12 | 826.15 |
| Crystal system, space group | Monoclinic, P21/c | Triclinic, P1 |
| Temperature (K) | 293 | 293 |
| a, b, c (Å) | 9.776 (2), 8.211 (2), 10.224 (2) | 9.194 (2), 9.458 (2), 11.176 (2) |
| α, β, γ (°) | 90, 99.75 (3), 90 | 101.15 (3), 101.76 (3), 106.17 (3) |
| V (Å3) | 808.8 (3) | 880.9 (4) |
| Z | 2 | 2 |
| Radiation type | Mo Kα | Mo Kα |
| µ (mm−1) | 21.82 | 20.06 |
| Crystal size (mm) | 0.09 × 0.07 × 0.04 | 0.18 × 0.03 × 0.03 |
| Data collection | ||
| Diffractometer | Nonius KappaCCD diffractometer | Nonius KappaCCD diffractometer |
| Absorption correction | Multi-scan (HKL SCALEPACK; Otwinowski & Minor, 1997) | Multi-scan (HKL SCALEPACK; Otwinowski & Minor, 1997) |
| Tmin, Tmax | 0.244, 0.476 | 0.123, 0.585 |
| No. of measured, independent and observed [I > 2σ(I)] reflections | 5650, 2924, 2670 | 10808, 6122, 4981 |
| Rint | 0.013 | 0.029 |
| (sin θ/λ)max (Å−1) | 0.757 | 0.758 |
| Refinement | ||
| R[F2 > 2σ(F2)], wR(F2), S | 0.019, 0.048, 1.06 | 0.028, 0.067, 1.01 |
| No. of reflections | 2924 | 6122 |
| No. of parameters | 119 | 264 |
| H-atom treatment | H-atom parameters not refined | H-atom parameters not refined |
| Δρmax, Δρmin (e Å−3) | 1.74, −1.85 | 1.73, −1.87 |
Computer programs: COLLECT (Nonius, 2002), HKL SCALEPACK (Otwinowski & Minor, 1997), HKL DENZO (Otwinowski & Minor, 1997) and SCALEPACK, SHELXS97 (Sheldrick, 1997), SHELXL97 (Sheldrick, 1997), ATOMS (Shape Software, 1999).
| Bi—O2i | 2.326 (2) | C1—O1 | 1.243 (4) |
| Bi—O5ii | 2.380 (2) | C1—O2 | 1.253 (4) |
| Bi—O3 | 2.435 (2) | C1—C1i | 1.553 (6) |
| Bi—OW1 | 2.449 (3) | C2—O3 | 1.246 (4) |
| Bi—O4ii | 2.527 (2) | C2—O4 | 1.257 (4) |
| Bi—O1 | 2.537 (2) | C2—C3 | 1.553 (4) |
| Bi—O6 | 2.614 (2) | C3—O6 | 1.245 (4) |
| Bi—OW2 | 2.658 (3) | C3—O5 | 1.254 (4) |
| Bi—O4iii | 2.663 (2) |
| Symmetry codes: (i) −x+1, −y, −z+2; (ii) −x, y−1/2, −z+3/2; (iii) x, −y+1/2, z+1/2. |
| Bi1—O12 | 2.320 (3) | Bi2—O8iv | 2.681 (3) |
| Bi1—O2 | 2.335 (3) | C1—O1 | 1.246 (5) |
| Bi1—O10 | 2.426 (3) | C1—O2 | 1.265 (5) |
| Bi1—O5i | 2.430 (3) | C1—C2 | 1.545 (6) |
| Bi1—O7i | 2.564 (3) | C2—O3 | 1.251 (5) |
| Bi1—OW3 | 2.577 (3) | C2—O4 | 1.253 (5) |
| Bi1—O3 | 2.597 (3) | C3—O5 | 1.247 (5) |
| Bi1—OW2 | 2.614 (4) | C3—O6 | 1.251 (5) |
| Bi1—O3ii | 2.763 (3) | C3—C4 | 1.557 (6) |
| Bi2—O6 | 2.349 (3) | C4—O8 | 1.248 (5) |
| Bi2—O1 | 2.398 (3) | C4—O7 | 1.251 (5) |
| Bi2—O9iii | 2.450 (3) | C5—O9 | 1.250 (5) |
| Bi2—O4 | 2.496 (3) | C5—O10 | 1.260 (5) |
| Bi2—OW1 | 2.498 (4) | C5—C6 | 1.548 (6) |
| Bi2—OW4 | 2.543 (4) | C6—O11 | 1.237 (5) |
| Bi2—O11iii | 2.553 (3) | C6—O12 | 1.262 (5) |
| Bi2—O8 | 2.577 (3) |
| Symmetry codes: (i) −x, −y+2, −z; (ii) −x, −y+1, −z; (iii) −x+1, −y+2, −z; (iv) −x, −y+2, −z−1. |

 Subscribe to Acta Crystallographica Section C: Structural Chemistry
Subscribe to Acta Crystallographica Section C: Structural Chemistry
The full text of this article is available to subscribers to the journal.
- Information on subscribing
- Sample issue
- Purchase subscription
- Reduced-price subscriptions
- If you have already subscribed, you may need to register

 journal menu
journal menu








![[Figure 1]](http://journals.iucr.org/c/issues/2003/12/00/ta1420/ta1420fig1thm.gif)
![[Figure 2]](http://journals.iucr.org/c/issues/2003/12/00/ta1420/ta1420fig2thm.gif)
![[Figure 3]](http://journals.iucr.org/c/issues/2003/12/00/ta1420/ta1420fig3thm.gif)
![[Figure 4]](http://journals.iucr.org/c/issues/2003/12/00/ta1420/ta1420fig4thm.gif)

 Subscribe to Acta Crystallographica Section C: Structural Chemistry
Subscribe to Acta Crystallographica Section C: Structural Chemistry



During work on a comprehensive review of the crystal chemistry and crystal structure types of complex metal oxalates (Fleck & Kolitsch, 2003), it was noted that data for bismuth(III) oxalates are very rare and no crystal structures have been reported. The only crystallographic study is that of Polla et al. (1984), who prepared and studied the space-group symmetries of two compounds, viz. monoclinic `bismuth hydrogen oxalate' (`Bi2(C2O4)3·H2C2O4'; P21/c) and triclinic `bismuth oxalate heptahydrate' (`Bi2(C2O4)3·7H2O'; P1 or P1), and who also investigated the thermal behavior of these compounds during heating. The formulae given were based on wet-chemical analyses of the Bi3+ and C2O42− contents (water was not determined directly) and a comparison between measured and calculated densities.
The present contribution reports structure determinations of both compounds, on the basis of single-crystal X-ray diffraction data collected at 293 K. The presently refined unit-cell parameters show good agreement with those given by Polla et al. (1984) [a = 9.77 (1) Å, b = 8.20 (1) Å, c = 10.23 (1) Å, β = 99.6 (1)° and V = 808.1 Å3 (monoclinic compound), and a = 9.18 (1) Å, b = 9.43 (1) Å, c = 11.17 (1) Å, α = 101.0 (1)°, β = 101.7 (1)°, γ = 106.3 (1)° and V = 876.7 Å3 (triclinic compound)]. The solutions of the crystal structures, however, led in both cases to a revision of the chemical formulae. The correct structural formula of `Bi2(C2O4)3·H2C2O4' (Polla et al., 1984) is Bi2(H2O)4(C2O4)3·2H2O, which can be simplified to Bi2(C2O4)3·6H2O. Thus, the monoclinic compound contains less oxalate per formula unit than previously reported. The correct formula of the compound `Bi2(C2O4)3·7H2O' (Polla et al., 1984) is Bi2(C2O4)3·8H2O, i.e. a formula with a higher water content.
The crystal structure of Bi2(C2O4)3·6H2O has space-group symmetry P21/c, and the asymmetric unit contains one Bi atom, three C atoms, six oxalate O atoms and three water O atoms; the H atoms of the latter could not be located. Two of the water O atoms, viz. OW1 and OW2, are bonded to the Bi atom (Table 2), whereas the third, viz. OW3, belongs to a `free' water molecule held in the structure only by medium- strong to weak hydrogen bonds [OW3···OW1 = 2.691 (4) Å and OW···O1 = 2.801 (4) Å] and two further possible bonds to OW2 (>2.85 Å). All detected atoms are in general positions.
The atomic arrangement in Bi2(C2O4)3·6H2O can be described as a three-dimensional network of Bi atoms, connected by tetradentate oxalate groups (Figs. 1 and 2). The Bi atom is surrounded by nine O atoms (including two aqua ligands, OW1 and OW2) within 2.67 Å in a fairly narrow range [2.326 (2) to 2.663 (2) Å]; the average Bi—O bond length is 2.510 Å. The BiO9 coordination polyhedron is relatively undistorted, and its coordination geometry indicates that the lone electron pair on the Bi3+ ion is not stereochemically active. The Bi atoms are connected via two tetradentate oxalate groups in two different directions, approximately along [411] and [575], thus creating a three-dimensional network. The three water molecules are all located in eight-shaped channels, approximately parallel to both [100] and [001]. The C2/C3- based oxalate group shows a very small deviation from planarity [1.2 (4)°].
A calculated X-ray powder diffraction pattern shows reasonable agreement with the measured data reported by Polla et al. (1984; see also ICDD-PDF 38–548) concerning the d values, but the agreement with the earlier reported intensities is relatively poor (possibly because of the effects of preferred orientation).
Bi2(C2O4)3·8H2O is triclinic, with space group P1. The asymmetric unit contains two Bi atoms, six C atoms, 12 oxalate O atoms and eight water molecules; the H atoms of the latter could not be located. Four of the eight water molecules, epresented by atoms OW1, OW2, OW3 and OW4, are bonded to atoms Bi1 and Bi2 (Table 4). The remaining four water molecules, represented by atoms OW5, OW6, OW7 and OW8, can be considered as `free' because they have no apparent bonds to the Bi atoms (all Bi—OW distances are greater than 4.3 Å). The OW8 site is split into two sites, viz. OW8A and OW8B, with a separation of 0.77 (2) Å and a refined occupancy ratio of 0.31 (6):0.69 (6). All detected atoms are in general positions, as in the hexahydrate. If the different characters of the two types of water molecules are accounted for, the structural formula of the compound can be written as Bi2(H2O)4(C2O4)3·4H2O.
The crystal structure of Bi2(C2O4)3·8H2O is similar to that of Bi2(C2O4)3·6H2O in that it is based on a three-dimensional network formed by the connection between tetradentate oxalate groups and Bi atoms (Figs. 3 and 4). Atom Bi1 is surrounded by nine O atoms (including two aqua ligands, OW2 and OW3) within 3.5 Å in a relatively large range [2.320 (3) to 2.763 (3) Å]; the average Bi1—O bond length is 2.514 Å. Atom Bi2 also has nine O ligands (including two aqua ligands, OW1 and OW4) within 3.5 Å, but in a slightly smaller range [2.349 (3) to 2.681 (3) Å]; the average Bi2—O bond length is 2.505 Å. Thus, the ligand environments of both Bi atoms are considerably more distorted than that of the Bi atom in Bi2(C2O4)3·6H2O (Table 2). Nonetheless, the lone electron pairs on both of the Bi3+ ions in the octahydrate are not stereochemically active, a situation that is comparable to the that in the hexahydrate. The eight water molecules are all located in voids and channels of the network, with the latter running parallel to all three major crystallographic axes.
The tetradentate oxalate group based on atoms C1 and C2 bridges atoms Bi1 and Bi2 roughly along [349]. The tetradentate oxalate group based on atoms C3 and C4 connects atoms Bi1 and Bi2 approximately along [584]. The tetradentate oxalate group based on atoms C5 and C6 connects atoms Bi1 and Bi2 approximately along [853]. All oxalate groups are non-planar, with maximum deviations from planarity of 7.1 (6) (C1/C2-based group), 4.1 (4) (C3/C4-based group) and 9.1 (4)° (C5/C6-based group). Probable hydrogen bonds are medium-strong to weak, with O···O distances all greater than 2.68 Å, with the single exception of the unusually short distance between atoms OW5 and OW8A [part of the split OW8 site; 2.543 (4) Å].
A calculated X-ray powder diffraction pattern shows reasonable agreement with the measured pattern reported by Polla et al. (1984; see also ICDD-PDF 38–549) concerning the d values, but considerably weaker intensities for all reflections with I less than I100.
Bi2(C2O4)3·6H2O and Bi2(C2O4)3·8H2O cannot be transformed directly into one another because their topologies are not similar, as shown in Figs. 1–4. This dissimilarity is also confirmed by analyses of the directions of the strongest interatomic connections and of the shortest Bi···Bi distances. In Bi2(C2O4)3·6H2O, the Bi sublattice can be described as a corrugated layer parallel to the (100) plane (cf. Fig. 2), with a Bi···Bi distance of 4.413 (1) Å. In contrast, in Bi2(C2O4)3·8H2O, each Bi1 (Bi2) atom has only one other Bi1 (Bi2) atom as its next cationic neighbour within 6.0 Å [Bi1···Bi1 = 4.534 (2) Å and Bi2···Bi2 = 4.431 (1) Å].
A noteworthy observation was made after the intensity data collections of both compounds; the previously colourless transparent thick-tabular crystal of the monoclinic oxalate then exhibited a pale-brown colour (darker brown at the crystal edges) and revealed an hour-glass internal structure, but without having lost its transparency. The triclinic oxalate exhibited a very similar pale-brown colour and was also still completely transparent. These observations can be tentatively explained by the genesis of point defects due to irradiation with the X-ray beam. A similar colour-change phenomenon was recently observed in our laboratory for the basic lead nitrate Pb13O8(OH)6(NO3)4, whose color turned from pale yellow before the data collection to brown–orange afterwards (Kolitsch & Tillmanns, 2003). Similarly, no loss of transparency was noted.