Buy article online - an online subscription or single-article purchase is required to access this article.

The previously unknown crystal structure of strontium magnesium phosphate, Sr
2+xMg
3-xP
4O
15 (
x 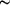
0.36), determined and refined from laboratory powder X-ray diffraction data, represents a new structure type. The title compound was synthesized by high-temperature solid-state reaction and it crystallizes in the orthorhombic space group
Cmcm. It was earlier thought to be stoichiometric Sr
2Mg
3P
4O
15, but our structural study indicates the nonstoichiometric composition. The asymmetric unit contains one Sr (site symmetry ..
m on special position 8
g), one
M (= Mg 64%/Sr 36%; site symmetry 2/
m.. on special position 4
b), one Mg (site symmetry 2.. on special position 8
e), two P (site symmetry
m.. on special position 8
f and site symmetry ..
m on special position 8
g), and six O sites [two on general positions 16
h, two on 8
g, one on 8
f and one on special position 4
c (site symmetry
m2
m)]. The nonstoichiometry is due to the mixing of magnesium and strontium ions on the
M site. The structure consists of three-dimensional networks of MgO
4 and PO
4 tetrahedra, and
MO
6 octahedra with the other strontium ions occupying the larger cavities surrounded by ten O atoms. All the polyhedra are connected by corner-sharing except the edge-sharing
MO
6 octahedra forming one-dimensional arrangements along [001].
Supporting information
Sr2+xMg3-xP4O15 (x~0.36) was synthesized by a
solid-state reaction from a mixture of high-purity SrCO3 (99.994%, Alfa
Aesar), MgO (99.0%, Yakuri Pure Chemicals) and (NH4)2HPO4 (99.0%, Junsei
Chemical) with a nominal composition of (Sr: Mg: P = 2: 3: 4). The mixture was
thoroughly ground in an agate mortar, dried, pressed into a pellet, heated in
air at 1303 K for 4 h, and again at 1323 K for 4 h with intermediate grinding
and pressing. The yield was about 90% by weight. The nonstoichiometric
composition of the major phase, Sr2+xMg3-xP4O15
(x~0.36), was determined later by the structural refinement, and the
minor impurity phases were determined to be Mg2P2O7 (Calvo, 1967)
and
Mg3P2O8 (Nord & Kierkegaard, 1968).
The powder X-ray diffraction (XRD) data were collected at room temperature on a
Bragg–Brentano diffractometer (Bruker AXS Advance D8) with a Cu X-ray tube, a
focusing primary Ge (111) monochromator (λ = 1.5406 Å) and a
position-sensitive Väntec detector with a 6° slit. Data acquisition covered
the angular range 8° ≤ 2θ ≤ 140° at a step width of 0.016682° and a
total measurement time of 40 h. The structure determination from the powder
XRD data was performed using a combination of the powder profile refinement
program GSAS (Larson & Von Dreele, 2000) and the single-crystal
structure refinement program CRYSTALS (Betteridge et al.,
2003).
For a three-dimensional view of the Fourier density maps, MCE was used
(Rohlíček & Hušák, 2007). The XRD pattern was indexed using the
program DICVOL91 (Boultif & Louër, 1991) run in
CRYSFIRE
(Shirley, 2002) via the positions of 20 diffraction peaks after
excluding the impurity peaks, resulting in an orthorhombic unit cell. The
systematic absences suggested three possible space groups: Cmc21,
C2cm and Cmcm. All of them would have resulted in basically the
same structure, thus the space group Cmcm with the highest symmetry was
chosen for the final refinement. LeBail fitting was carried out for the
previously unknown phase of Sr2+xMg3-xP4O15, while
Rietveld fitting was carried out for the two known impurity phases.
The structure determination was performed in the same way as in our previous
work (Lee & Hong, 2008) where the details were described. At the
beginning, a
structural model with only a dummy atom at an arbitrary position in the unit
cell was used. Structure factors were extracted from the powder data, then
direct methods were used for the initial solution of the structure using
SHELXS (Sheldrick, 2008) run in CRYSTALS, which yielded
several
metal positions. However, not all the atoms could be identified at once. The
partial model at this stage replaced the initial dummy-atom model, and was
used for a LeBail fit in GSAS. Then, improved structure factors were
extracted, which were used for the improved data in the refinement in
CRYSTALS. These processes were iterated until a complete and
satisfactory structural model was obtained. Finally, Rietveld refinement was
employed to complete the structure determination. Up to this step, a
stoichiometric composition of Sr2Mg3P4O15 was assumed for the unknown
phase, and indeed the crystallographic sites ratio seemed to conform to the
stoichiometry. However, the thermal parameter of the Mg1 site went to a
very small value, and Mg1—O distances (circa 2.3 Å) were longer
than the expected (2.10 Å), but much shorter than the 2.56 Å expected for
d(Sr—O) from ionic radii (Shannon, 1976). Therefore, it was
assumed
that some magnesium is substituted by strontium in the Mg1 site, and Sr2 and
Mg1 occupancies were refined with a constraint that the thermal
parameters for M (Mg1/Sr2) and Mg2 sites were the same, resulting
in a dramatic improvement in refinement (wRp factors from 16.8 to
6.3%) with reasonable thermal parameters. Lowering the symmetry to
Cmc21 or C2cm did not separate the problematic Mg1 site, and
thus should not make any difference to this outcome. For the impurity phases,
only the cell parameters and scale factors were refined while the other
variables were fixed in the final refinement. The overall fit (wRp =
6.3%) is shown in Fig. 1.
For all compounds, data collection: COMMANDER (Bruker, 2003); cell refinement: GSAS (Larson & Von Dreele, 2000); data reduction: EVA (Bruker, 2003); program(s) used to solve structure: SHELXS (Sheldrick, 2008) and CRYSTALS (Betteridge et al., 2003); program(s) used to refine structure: GSAS (Larson & Von Dreele, 2000); molecular graphics: ATOMS (Dowty, 2000); software used to prepare material for publication: GSAS (Larson & Von Dreele, 2000).
(phase_1) distrontium dimagnesium pentadecaoxidotetraphosphate
top
Crystal data top
| Sr2.36Mg2.64P4O15 | Z = 4 |
| Mr = 634.65 | F(000) = 1204.4 |
| Orthorhombic, Cmcm | Dx = 3.490 Mg m−3 |
| Hall symbol: -C 2c 2 | Cu Kα1 radiation, λ = 1.5406 Å |
| a = 14.27366 (9) Å | T = 296 K |
| b = 11.75678 (7) Å | white |
| c = 7.19753 (4) Å | flat sheet, 20 × 20 mm |
| V = 1207.83 (1) Å3 | Specimen preparation: Prepared at 296 K |
Data collection top
Bruker D8 Advance
diffractometer | Data collection mode: reflection |
| Radiation source: sealed X-ray tube, Bruker Cu Ceramic X-ray tube | Scan method: step |
| Ge 111 monochromator | 2θmin = 8.0°, 2θmax = 140.422°, 2θstep = 0.017° |
| Specimen mounting: packed powder pellet | |
Refinement top
| Least-squares matrix: full | 7939 data points |
| Rp = 0.048 | Profile function: CW Profile function number 4 with 18 terms
Pseudovoigt profile coefficients as parameterized in
Thompson et al.
[Thompson, P., Cox, D. E. & Hastings, J. B. (1987). J. Appl.
Cryst. 20, 79–83].
Asymmetry correction of Finger et al.
[Finger, L. W., Cox, D. E. & Jephcoat, A. P. (1994). J. Appl.
Cryst. 27, 892–900].
Microstrain broadening by Stephens
[Stephens, P. W. (1999). J. Appl. Cryst. 32, 281–289].
#1(GU) = 101.674 #2(GV) = -32.707 #3(GW) = 2.792
#4(GP) = 11.704 #5(LX) = 5.714 #6(ptec) = 0.00
#7(trns) = 0.00 #8(shft) = 3.1799 #9(sfec) = 0.00
#10(S/L) = 0.0087 #11(H/L) = 0.0005 #12(eta) = 0.7500
#13(S400 ) = 0.0E+00 #14(S040 ) = 0.0E+00 #15(S004 ) = 0.0E+00
#16(S220 ) = 0.0E+00 #17(S202 ) = 0.0E+00 #18(S022 ) = 0.0E+00
Peak tails are ignored where the intensity is below 0.0020 times the peak
Aniso. broadening axis 0.0 0.0 1.0 |
| Rwp = 0.063 | 81 parameters |
| Rexp = 0.025 | 0 restraints |
| RBragg = 0.047 | Weighting scheme based on measured s.u.'s |
| R(F) = 0.028 | (Δ/σ)max = 0.02 |
| R(F2) = 0.04372 | Background function: GSAS Background function number 1 with 36 terms.
Shifted Chebyshev function of 1st kind
1: 667.815 2: -121.026 3: 250.928 4: -108.029
5: 130.095 6: -120.393 7: 81.5238 8: -66.4354
9: 31.4946 10: -19.2674 11: 16.0150 12: -21.1906
13: 24.6787 14: -7.73173 15: 5.82669 16: 7.16351
17: 9.66948 18: 8.02466 19: 5.65618 20: 4.74674
21: -11.8793 22: -0.873263 23: -2.40368 24: -9.55052
25: -6.36605 26: 6.28370 27: -8.07052 28: 6.33955
29: -12.6008 30: 3.68005 31: 5.95000 32: 7.62516
33: 1.58465 34: 5.63269 35: -2.90398 36: 14.7130 |
| χ2 = 6.250 | |
Crystal data top
| Sr2.36Mg2.64P4O15 | V = 1207.83 (1) Å3 |
| Mr = 634.65 | Z = 4 |
| Orthorhombic, Cmcm | Cu Kα1 radiation, λ = 1.5406 Å |
| a = 14.27366 (9) Å | T = 296 K |
| b = 11.75678 (7) Å | flat sheet, 20 × 20 mm |
| c = 7.19753 (4) Å | |
Data collection top
Bruker D8 Advance
diffractometer | Scan method: step |
| Specimen mounting: packed powder pellet | 2θmin = 8.0°, 2θmax = 140.422°, 2θstep = 0.017° |
| Data collection mode: reflection | |
Refinement top
| Rp = 0.048 | R(F2) = 0.04372 |
| Rwp = 0.063 | χ2 = 6.250 |
| Rexp = 0.025 | 7939 data points |
| RBragg = 0.047 | 81 parameters |
| R(F) = 0.028 | 0 restraints |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2) top| | x | y | z | Uiso*/Ueq | Occ. (<1) |
| Sr1 | 0.16729 (5) | 0.29123 (6) | 0.25 | 0.0157 (2)* | |
| Sr2 | 0.0 | 0.5 | 0.0 | 0.0185 (4)* | 0.3571 (23) |
| Mg1 | 0.0 | 0.5 | 0.0 | 0.0185 (4)* | 0.6429 (23) |
| Mg2 | 0.16691 (18) | 0.0 | 0.0 | 0.0185 (4)* | |
| P1 | 0.0 | 0.80342 (16) | 0.0344 (3) | 0.0148 (4)* | |
| P2 | 0.19114 (12) | 0.57060 (18) | 0.25 | 0.0148 (4)* | |
| O1 | 0.0 | 0.6879 (4) | −0.0498 (5) | 0.0250 (5)* | |
| O2 | 0.0979 (3) | 0.5046 (4) | 0.25 | 0.0250 (5)* | |
| O3 | 0.08811 (19) | 0.1331 (2) | 0.0171 (4) | 0.0250 (5)* | |
| O4 | 0.2279 (3) | −0.0215 (4) | 0.25 | 0.0250 (5)* | |
| O5 | 0.0 | 0.7839 (6) | 0.25 | 0.0250 (5)* | |
| O6 | 0.2059 (2) | 0.6400 (3) | 0.0769 (4) | 0.0250 (5)* | |
Atomic displacement parameters (Å2) top| | U11 | U22 | U33 | U12 | U13 | U23 |
| ? | ? | ? | ? | ? | ? | ? |
Geometric parameters (Å, º) top
| Sr1—O1i | 2.7998 (19) | P1—O3ii | 1.509 (3) |
| Sr1—O1ii | 2.7998 (19) | P1—O3v | 1.509 (3) |
| Sr1—O2 | 2.697 (4) | P1—O5 | 1.5684 (19) |
| Sr1—O3 | 2.747 (3) | P2—Mg2iv | 2.834 (2) |
| Sr1—O3iii | 2.747 (3) | P2—Mg2xv | 2.834 (2) |
| Sr1—O4iv | 2.662 (4) | P2—O2 | 1.541 (4) |
| Sr1—O6i | 2.548 (3) | P2—O4iv | 1.584 (5) |
| Sr1—O6v | 2.548 (3) | P2—O6 | 1.504 (3) |
| Sr1—O6vi | 2.827 (3) | P2—O6iii | 1.504 (3) |
| Sr1—O6vii | 2.827 (3) | O1—Sr1ix | 2.7998 (19) |
| Sr2—O1 | 2.238 (4) | O1—Sr1x | 2.7998 (19) |
| Sr2—O1ii | 2.238 (4) | O1—Sr2 | 2.238 (4) |
| Sr2—O2 | 2.279 (3) | O1—Mg1 | 2.238 (4) |
| Sr2—O2viii | 2.279 (3) | O1—P1 | 1.487 (4) |
| Sr2—O2ix | 2.279 (3) | O2—Sr1 | 2.697 (4) |
| Sr2—O2x | 2.279 (3) | O2—Sr2 | 2.279 (3) |
| Sr2—O6 | 3.414 (3) | O2—Sr2i | 2.279 (3) |
| Sr2—O6viii | 3.414 (3) | O2—Mg1 | 2.279 (3) |
| Sr2—O6ii | 3.414 (3) | O2—Mg1i | 2.279 (3) |
| Sr2—O6v | 3.414 (3) | O2—P2 | 1.541 (4) |
| Mg1—O1 | 2.238 (4) | O3—Sr1 | 2.747 (3) |
| Mg1—O1ii | 2.238 (4) | O3—Mg2 | 1.931 (3) |
| Mg1—O2 | 2.279 (3) | O3—P1ii | 1.509 (3) |
| Mg1—O2viii | 2.279 (3) | O4—Sr1vi | 2.662 (4) |
| Mg1—O2ix | 2.279 (3) | O4—Mg2 | 2.015 (2) |
| Mg1—O2x | 2.279 (3) | O4—Mg2xvi | 2.015 (2) |
| Mg2—P2vi | 2.834 (2) | O4—P2vi | 1.584 (5) |
| Mg2—P2xi | 2.834 (2) | O5—P1 | 1.5684 (19) |
| Mg2—O3 | 1.931 (3) | O5—P1xvii | 1.5684 (19) |
| Mg2—O3xii | 1.931 (3) | O6—Sr1ix | 2.548 (3) |
| Mg2—O4 | 2.015 (2) | O6—Sr1iv | 2.827 (3) |
| Mg2—O4xiii | 2.015 (2) | O6—Sr2 | 3.414 (3) |
| Mg2—O6vi | 2.512 (3) | O6—Mg2iv | 2.512 (3) |
| Mg2—O6xiv | 2.512 (3) | O6—P2 | 1.504 (3) |
| P1—O1 | 1.487 (4) | | |
| | | |
| O1i—Sr1—O1ii | 61.95 (13) | O1—Mg1—O2x | 84.08 (13) |
| O1i—Sr1—O2 | 66.74 (12) | O1ii—Mg1—O2 | 84.08 (13) |
| O1i—Sr1—O3 | 91.29 (10) | O1ii—Mg1—O2viii | 84.08 (13) |
| O1i—Sr1—O3xviii | 52.72 (9) | O1ii—Mg1—O2ix | 95.92 (13) |
| O1i—Sr1—O4iv | 114.01 (11) | O1ii—Mg1—O2x | 95.92 (13) |
| O1i—Sr1—O6i | 71.43 (9) | O2—Mg1—O2viii | 75.61 (16) |
| O1i—Sr1—O6v | 129.19 (10) | O2—Mg1—O2ix | 104.39 (16) |
| O1i—Sr1—O6vi | 145.90 (11) | O2—Mg1—O2x | 180.0 |
| O1i—Sr1—O6xix | 111.99 (10) | O2viii—Mg1—O2ix | 179.9802 |
| O1ii—Sr1—O2 | 66.74 (12) | O2viii—Mg1—O2x | 104.39 (16) |
| O1ii—Sr1—O3 | 52.72 (9) | O2ix—Mg1—O2x | 75.61 (16) |
| O1ii—Sr1—O3xviii | 91.29 (10) | O3—Mg2—O3xii | 108.7 (2) |
| O1ii—Sr1—O4iv | 114.01 (11) | O3—Mg2—O4 | 107.24 (14) |
| O1ii—Sr1—O6i | 129.19 (10) | O3—Mg2—O4xiii | 101.93 (15) |
| O1ii—Sr1—O6v | 71.43 (9) | O3xii—Mg2—O4 | 101.93 (15) |
| O1ii—Sr1—O6vi | 111.99 (10) | O3xii—Mg2—O4xiii | 107.24 (14) |
| O1ii—Sr1—O6xix | 145.90 (11) | O4—Mg2—O4xiii | 128.8 (3) |
| O2—Sr1—O3 | 118.58 (9) | O1—P1—O3ii | 110.58 (17) |
| O2—Sr1—O3xviii | 118.58 (9) | O1—P1—O3v | 110.58 (17) |
| O2—Sr1—O4iv | 55.77 (13) | O1—P1—O5 | 105.6 (3) |
| O2—Sr1—O6i | 77.54 (8) | O3ii—P1—O3v | 112.9 (3) |
| O2—Sr1—O6v | 77.54 (8) | O3ii—P1—O5 | 108.4 (2) |
| O2—Sr1—O6vi | 145.12 (9) | O3v—P1—O5 | 108.4 (2) |
| O2—Sr1—O6xix | 145.12 (9) | O2—P2—O4iv | 106.6 (3) |
| O3—Sr1—O3xviii | 75.21 (12) | O2—P2—O6 | 113.22 (17) |
| O3—Sr1—O4iv | 142.30 (6) | O2—P2—O6xviii | 113.22 (17) |
| O3—Sr1—O6i | 150.15 (10) | O4iv—P2—O6 | 105.57 (16) |
| O3—Sr1—O6v | 74.96 (8) | O4iv—P2—O6xviii | 105.57 (16) |
| O3—Sr1—O6vi | 64.44 (8) | O6—P2—O6xviii | 111.9 (3) |
| O3—Sr1—O6xix | 96.12 (8) | Sr1ix—O1—Sr1x | 117.05 (13) |
| O3xviii—Sr1—O4iv | 142.30 (6) | Sr1ix—O1—Sr2 | 99.73 (11) |
| O3xviii—Sr1—O6i | 74.96 (8) | Sr1ix—O1—Mg1 | 99.73 (11) |
| O3xviii—Sr1—O6v | 150.15 (10) | Sr1ix—O1—P1 | 97.45 (13) |
| O3xviii—Sr1—O6vi | 96.12 (8) | Sr1x—O1—Sr2 | 99.73 (11) |
| O3xviii—Sr1—O6xix | 64.44 (8) | Sr1x—O1—Mg1 | 99.73 (11) |
| O4iv—Sr1—O6i | 67.41 (7) | Sr1x—O1—P1 | 97.45 (13) |
| O4iv—Sr1—O6v | 67.41 (7) | Sr2—O1—P1 | 146.7 (3) |
| O4iv—Sr1—O6vi | 99.23 (11) | Mg1—O1—P1 | 146.7 (3) |
| O4iv—Sr1—O6xix | 99.23 (11) | Sr1—O2—Sr2 | 101.73 (13) |
| O6i—Sr1—O6v | 134.82 (14) | Sr1—O2—Sr2i | 101.73 (13) |
| O6i—Sr1—O6vi | 117.91 (7) | Sr1—O2—Mg1 | 101.73 (13) |
| O6i—Sr1—O6xix | 69.77 (10) | Sr1—O2—Mg1i | 101.73 (13) |
| O6v—Sr1—O6vi | 69.77 (10) | Sr1—O2—P2 | 98.7 (2) |
| O6v—Sr1—O6xix | 117.91 (7) | Sr2—O2—Sr2i | 104.32 (16) |
| O6vi—Sr1—O6xix | 52.31 (12) | Sr2—O2—Mg1i | 104.32 (16) |
| O1—Sr2—O1ii | 179.9657 | Sr2—O2—P2 | 122.79 (12) |
| O1—Sr2—O2 | 95.92 (13) | Sr2i—O2—Mg1 | 104.32 (16) |
| O1—Sr2—O2viii | 95.92 (13) | Sr2i—O2—P2 | 122.79 (12) |
| O1—Sr2—O2ix | 84.08 (13) | Mg1—O2—Mg1i | 104.32 (16) |
| O1—Sr2—O2x | 84.08 (13) | Mg1—O2—P2 | 122.79 (12) |
| O1ii—Sr2—O2 | 84.08 (13) | Mg1i—O2—P2 | 122.79 (12) |
| O1ii—Sr2—O2viii | 84.08 (13) | Sr1—O3—Mg2 | 110.35 (13) |
| O1ii—Sr2—O2ix | 95.92 (13) | Sr1—O3—P1ii | 99.10 (16) |
| O1ii—Sr2—O2x | 95.92 (13) | Mg2—O3—P1ii | 150.5 (2) |
| O2—Sr2—O2viii | 75.61 (16) | Sr1vi—O4—Mg2 | 110.29 (12) |
| O2—Sr2—O2ix | 104.39 (16) | Sr1vi—O4—Mg2xvi | 110.29 (12) |
| O2—Sr2—O2x | 180.0 | Sr1vi—O4—P2vi | 98.9 (2) |
| O2viii—Sr2—O2ix | 179.9802 | Mg2—O4—Mg2xvi | 126.5 (3) |
| O2viii—Sr2—O2x | 104.39 (16) | Mg2—O4—P2vi | 103.25 (15) |
| O2ix—Sr2—O2x | 75.61 (16) | Mg2xvi—O4—P2vi | 103.25 (15) |
| O1—Mg1—O1ii | 179.9657 | P1—O5—P1xx | 163.2 (5) |
| O1—Mg1—O2 | 95.92 (13) | Sr1ix—O6—Sr1iv | 110.23 (10) |
| O1—Mg1—O2viii | 95.92 (13) | Sr1ix—O6—P2 | 155.0 (2) |
| O1—Mg1—O2ix | 84.08 (13) | Sr1iv—O6—P2 | 93.78 (16) |
| Symmetry codes: (i) x, −y+1, z+1/2; (ii) −x, −y+1, −z; (iii) x, y, −z+1/2; (iv) −x+1/2, y+1/2, z; (v) x, −y+1, −z; (vi) −x+1/2, y−1/2, z; (vii) −x−1/2, y−3/2, −z+1/2; (viii) −x, y, z; (ix) x, −y+1, z−1/2; (x) −x, −y+1, z−1/2; (xi) −x+1/2, −y+1/2, z−1/2; (xii) x, −y, −z; (xiii) x, −y, z−1/2; (xiv) −x−1/2, −y−1/2, −z; (xv) −x+1/2, −y+1/2, z+1/2; (xvi) x, −y, z+1/2; (xvii) −x, y, −z+1/2; (xviii) x, y, −z+3/2; (xix) −x+1/2, y−1/2, −z+3/2; (xx) −x−1, y−1, −z+1/2. |
(phase_2) dimagnesium heptaoxidodiphosphate
top
Crystal data top
| Mg2P2O7 | Z = 4 |
| Mr = 222.56 | F(000) = 440.0 |
| Monoclinic, P21/c | Dx = 3.105 Mg m−3 |
| Hall symbol: -P 2ybc | Cu Kα1 radiation, λ = 1.5406 Å |
| a = 6.9443 (4) Å | T = 296 K |
| b = 8.2861 (4) Å | white |
| c = 9.0438 (5) Å | flat sheet, 20 × 20 mm |
| β = 113.816 (3)° | Specimen preparation: Prepared at 296 K |
| V = 476.08 (5) Å3 | |
Data collection top
Bruker D8 Advance
diffractometer | Data collection mode: reflection |
| Radiation source: sealed X-ray tube, Bruker Cu Ceramic X-ray tube | Scan method: step |
| Ge 111 monochromator | 2θmin = 8.0°, 2θmax = 140.422°, 2θstep = 0.017° |
| Specimen mounting: packed powder pellet | |
Refinement top
| Least-squares matrix: full | 7939 data points |
| Rp = 0.048 | Profile function: CW Profile function number 4 with 21 terms
Pseudovoigt profile coefficients as parameterized in
Thompson et al.
[Thompson, P., Cox, D. E. & Hastings, J. B. (1987). J. Appl.
Cryst. 20, 79–83].
Asymmetry correction of Finger et al.
[Finger, L. W., Cox, D. E. & Jephcoat, A. P. (1994). J. Appl.
Cryst. 27, 892–900].
Microstrain broadening by Stephens
[Stephens, P. W. (1999). J. Appl. Cryst. 32, 281–289].
#1(GU) = 178.981 #2(GV) = -107.291 #3(GW) = 12.592
#4(GP) = 19.425 #5(LX) = 3.011 #6(ptec) = 0.00
#7(trns) = 0.00 #8(shft) = 3.1799 #9(sfec) = 0.00
#10(S/L) = 0.0005 #11(H/L) = 0.0005 #12(eta) = 0.7500
#13(S400 ) = 0.0E+00 #14(S040 ) = 0.0E+00 #15(S004 ) = 0.0E+00
#16(S220 ) = 0.0E+00 #17(S202 ) = 0.0E+00 #18(S022 ) = 0.0E+00
#19(S301 ) = 0.0E+00 #20(S103 ) = 0.0E+00 #21(S121 ) = 0.0E+00
Peak tails are ignored where the intensity is below 0.0020 times the peak
Aniso. broadening axis 0.0 0.0 1.0 |
| Rwp = 0.063 | 81 parameters |
| Rexp = 0.025 | 0 restraints |
| RBragg = 0.047 | Weighting scheme based on measured s.u.'s |
| R(F) = 0.047 | (Δ/σ)max = 0.02 |
| R(F2) = 0.04372 | Background function: GSAS Background function number 1 with 36 terms.
Shifted Chebyshev function of 1st kind
1: 667.815 2: -121.026 3: 250.928 4: -108.029
5: 130.095 6: -120.393 7: 81.5238 8: -66.4354
9: 31.4946 10: -19.2674 11: 16.0150 12: -21.1906
13: 24.6787 14: -7.73173 15: 5.82669 16: 7.16351
17: 9.66948 18: 8.02466 19: 5.65618 20: 4.74674
21: -11.8793 22: -0.873263 23: -2.40368 24: -9.55052
25: -6.36605 26: 6.28370 27: -8.07052 28: 6.33955
29: -12.6008 30: 3.68005 31: 5.95000 32: 7.62516
33: 1.58465 34: 5.63269 35: -2.90398 36: 14.7130 |
| χ2 = 6.250 | |
Crystal data top
| Mg2P2O7 | β = 113.816 (3)° |
| Mr = 222.56 | V = 476.08 (5) Å3 |
| Monoclinic, P21/c | Z = 4 |
| a = 6.9443 (4) Å | Cu Kα1 radiation, λ = 1.5406 Å |
| b = 8.2861 (4) Å | T = 296 K |
| c = 9.0438 (5) Å | flat sheet, 20 × 20 mm |
Data collection top
Bruker D8 Advance
diffractometer | Scan method: step |
| Specimen mounting: packed powder pellet | 2θmin = 8.0°, 2θmax = 140.422°, 2θstep = 0.017° |
| Data collection mode: reflection | |
Refinement top
| Rp = 0.048 | R(F2) = 0.04372 |
| Rwp = 0.063 | χ2 = 6.250 |
| Rexp = 0.025 | 7939 data points |
| RBragg = 0.047 | 81 parameters |
| R(F) = 0.047 | 0 restraints |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2) top| | x | y | z | Uiso*/Ueq | |
| Mg1 | 0.24957 | 0.92491 | 0.12118 | 0.02084* | |
| Mg2 | 0.69861 | 0.43311 | 0.82859 | 0.02084* | |
| P1 | 0.94785 | 0.76438 | 0.76493 | 0.02084* | |
| P2 | 0.52451 | 0.77297 | 0.46834 | 0.02084* | |
| O1 | 0.72902 | 0.83262 | 0.59014 | 0.02084* | |
| O2 | 0.37623 | 0.76109 | 0.55127 | 0.02084* | |
| O3 | 1.12756 | 0.76909 | 0.69986 | 0.02084* | |
| O4 | 1.01129 | 0.90711 | 0.89533 | 0.02084* | |
| O5 | 0.92364 | 0.5963 | 0.82874 | 0.02084* | |
| O6 | 0.47096 | 0.89728 | 0.32033 | 0.02084* | |
| O7 | 0.59983 | 0.60601 | 0.4192 | 0.02084* | |
Atomic displacement parameters (Å2) top| | U11 | U22 | U33 | U12 | U13 | U23 |
| ? | ? | ? | ? | ? | ? | ? |
Geometric parameters (Å, º) top
| Mg1—O2i | 1.9994 (1) | O1—P1 | 1.7854 (1) |
| Mg1—O3ii | 2.0725 (1) | O1—P2 | 1.4870 (1) |
| Mg1—O4iii | 2.0499 (1) | O2—Mg1viii | 1.9994 (1) |
| Mg1—O4iv | 2.2412 (1) | O2—Mg2x | 1.9845 (1) |
| Mg1—O6 | 1.8503 (1) | O2—P2 | 1.5021 (1) |
| Mg1—O7v | 1.9461 (1) | O3—Mg1xi | 2.0725 (1) |
| Mg2—O2vi | 1.9845 (1) | O3—Mg2xii | 1.9030 (1) |
| Mg2—O3vii | 1.9030 (1) | O3—P1 | 1.5806 (1) |
| Mg2—O5 | 2.0661 (1) | O4—Mg1xiii | 2.0499 (1) |
| Mg2—O6viii | 2.0939 (1) | O4—Mg1iv | 2.2412 (1) |
| Mg2—O7ix | 2.3814 (1) | O4—P1 | 1.6013 (1) |
| P1—O1 | 1.7854 (1) | O5—Mg2 | 2.0661 (1) |
| P1—O3 | 1.5806 (1) | O5—P1 | 1.5425 (1) |
| P1—O4 | 1.6013 (1) | O6—Mg1 | 1.8503 (1) |
| P1—O5 | 1.5425 (1) | O6—Mg2i | 2.0939 (1) |
| P2—O1 | 1.4870 (1) | O6—P2 | 1.6091 (1) |
| P2—O2 | 1.5021 (1) | O7—Mg1xiv | 1.9461 (1) |
| P2—O6 | 1.6091 (1) | O7—Mg2ix | 2.3814 (1) |
| P2—O7 | 1.6040 (1) | O7—P2 | 1.6040 (1) |
| | | |
| O2xv—Mg1—O3xvi | 78.680 (4) | O1—P1—O5 | 115.771 (2) |
| O2xv—Mg1—O4iii | 84.872 (3) | O3—P1—O4 | 103.426 (3) |
| O2xv—Mg1—O4iv | 154.7676 (11) | O3—P1—O5 | 112.5380 (13) |
| O2xv—Mg1—O6 | 85.052 (3) | O4—P1—O5 | 115.496 (4) |
| O2xv—Mg1—O7v | 101.344 (4) | O1—P2—O2 | 107.107 (5) |
| O3xvi—Mg1—O4iii | 90.507 (3) | O1—P2—O6 | 103.548 (3) |
| O3xvi—Mg1—O4iv | 94.190 (4) | O1—P2—O7 | 99.856 (3) |
| O3xvi—Mg1—O6 | 82.185 (3) | O2—P2—O6 | 118.856 (3) |
| O3xvi—Mg1—O7v | 171.0005 (5) | O2—P2—O7 | 116.6010 (19) |
| O4iii—Mg1—O4iv | 70.899 (3) | O6—P2—O7 | 108.259 (4) |
| O4iii—Mg1—O6 | 168.5470 (8) | P1—O1—P2 | 140.125 (3) |
| O4iii—Mg1—O7v | 98.467 (3) | Mg1xv—O2—Mg2x | 97.738 (4) |
| O4iv—Mg1—O6 | 118.245 (3) | Mg1xviii—O2—P2 | 133.3317 (16) |
| O4iv—Mg1—O7v | 89.414 (4) | Mg2x—O2—P2 | 127.599 (2) |
| O6—Mg1—O7v | 88.844 (3) | Mg1xix—O3—Mg2xii | 97.926 (4) |
| O2vi—Mg2—O3vii | 83.208 (4) | Mg1xx—O3—P1 | 127.0411 (19) |
| O2vi—Mg2—O5 | 146.6086 (16) | Mg2xii—O3—P1 | 135.016 (2) |
| O2vi—Mg2—O6xvii | 98.094 (4) | Mg1xiii—O4—Mg1iv | 109.101 (3) |
| O2vi—Mg2—O7ix | 95.548 (4) | Mg1xiii—O4—P1 | 131.173 (2) |
| O3vii—Mg2—O5 | 86.937 (4) | Mg1iv—O4—P1 | 115.787 (3) |
| O3vii—Mg2—O6xvii | 170.2380 (6) | Mg2—O5—P1 | 141.9393 (17) |
| O3vii—Mg2—O7ix | 97.705 (4) | Mg1—O6—Mg2xxi | 105.681 (3) |
| O5—Mg2—O6xvii | 96.931 (4) | Mg1—O6—P2 | 135.320 (2) |
| O5—Mg2—O7ix | 117.404 (3) | Mg2xxii—O6—P2 | 118.845 (3) |
| O6xvii—Mg2—O7ix | 72.552 (3) | Mg1xiv—O7—Mg2ix | 92.744 (3) |
| O1—P1—O3 | 100.385 (5) | Mg1xiv—O7—P2 | 167.7228 (6) |
| O1—P1—O4 | 107.510 (3) | Mg2ix—O7—P2 | 96.943 (3) |
| Symmetry codes: (i) x, −y+3/2, z−1/2; (ii) x−1, −y+3/2, z−1/2; (iii) x−1, y, z−1; (iv) −x+1, −y+2, −z+1; (v) −x+1, y+1/2, −z+1/2; (vi) −x+1, y−1/2, −z+3/2; (vii) −x+2, y−1/2, −z+3/2; (viii) x, −y+3/2, z+1/2; (ix) −x+1, −y+1, −z+1; (x) −x+1, y+1/2, −z+3/2; (xi) x+1, −y+3/2, z+1/2; (xii) −x+2, y+1/2, −z+3/2; (xiii) x+1, y, z+1; (xiv) −x+1, y−1/2, −z+1/2; (xv) x, −y+5/2, z+1/2; (xvi) x−1, −y+5/2, z+1/2; (xvii) x, −y+5/2, z+3/2; (xviii) x−1, −y+3/2, z+3/2; (xix) x+1, −y+5/2, z+1/2; (xx) x, −y+3/2, z+3/2; (xxi) x, −y+5/2, z−1/2; (xxii) x−1, −y+3/2, z+1/2. |
(phase_3) trimagnesium ocyaoxidodiphosphate
top
Crystal data top
| Mg3P2O8 | Z = 2 |
| Mr = 262.87 | F(000) = 260.0 |
| Monoclinic, P21/n | Dx = 2.745 Mg m−3 |
| Hall symbol: -P 2yn | Cu Kα1 radiation, λ = 1.5406 Å |
| a = 7.594 (3) Å | T = 296 K |
| b = 8.282 (8) Å | white |
| c = 5.071 (4) Å | flat sheet, 20 × 20 mm |
| β = 94.01 (6)° | Specimen preparation: Prepared at 296 K |
| V = 318.1 (4) Å3 | |
Data collection top
Bruker D8 Advance
diffractometer | Data collection mode: reflection |
| Radiation source: sealed X-ray tube, Bruker Cu Ceramic X-ray tube | Scan method: step |
| Ge 111 monochromator | 2θmin = 8.0°, 2θmax = 140.422°, 2θstep = 0.017° |
| Specimen mounting: packed powder pellet | |
Refinement top
| Least-squares matrix: full | 7939 data points |
| Rp = 0.048 | Profile function: CW Profile function number 4 with 21 terms
Pseudovoigt profile coefficients as parameterized in
Thompson et al.
[Thompson, P., Cox, D. E. & Hastings, J. B. (1987). J. Appl.
Cryst. 20, 79–83].
Asymmetry correction of Finger et al.
[Finger, L. W., Cox, D. E. & Jephcoat, A. P. (1994). J. Appl.
Cryst. 27, 892–900].
Microstrain broadening by Stephens
[Stephens, P. W. (1999). J. Appl. Cryst. 32, 281–289].
#1(GU) = 0.000 #2(GV) = 61.116 #3(GW) = -3.576
#4(GP) = 8.238 #5(LX) = 9.783 #6(ptec) = 0.00
#7(trns) = 0.00 #8(shft) = 3.1799 #9(sfec) = 0.00
#10(S/L) = 0.0005 #11(H/L) = 0.0005 #12(eta) = 0.7500
#13(S400 ) = 0.0E+00 #14(S040 ) = 0.0E+00 #15(S004 ) = 0.0E+00
#16(S220 ) = 0.0E+00 #17(S202 ) = 0.0E+00 #18(S022 ) = 0.0E+00
#19(S301 ) = 0.0E+00 #20(S103 ) = 0.0E+00 #21(S121 ) = 0.0E+00
Peak tails are ignored where the intensity is below 0.0020 times the peak
Aniso. broadening axis 0.0 0.0 1.0 |
| Rwp = 0.063 | 81 parameters |
| Rexp = 0.025 | 0 restraints |
| RBragg = 0.047 | Weighting scheme based on measured s.u.'s |
| R(F) = 0.049 | (Δ/σ)max = 0.02 |
| R(F2) = 0.04372 | Background function: GSAS Background function number 1 with 36 terms.
Shifted Chebyshev function of 1st kind
1: 667.815 2: -121.026 3: 250.928 4: -108.029
5: 130.095 6: -120.393 7: 81.5238 8: -66.4354
9: 31.4946 10: -19.2674 11: 16.0150 12: -21.1906
13: 24.6787 14: -7.73173 15: 5.82669 16: 7.16351
17: 9.66948 18: 8.02466 19: 5.65618 20: 4.74674
21: -11.8793 22: -0.873263 23: -2.40368 24: -9.55052
25: -6.36605 26: 6.28370 27: -8.07052 28: 6.33955
29: -12.6008 30: 3.68005 31: 5.95000 32: 7.62516
33: 1.58465 34: 5.63269 35: -2.90398 36: 14.7130 |
| χ2 = 6.250 | |
Crystal data top
| Mg3P2O8 | β = 94.01 (6)° |
| Mr = 262.87 | V = 318.1 (4) Å3 |
| Monoclinic, P21/n | Z = 2 |
| a = 7.594 (3) Å | Cu Kα1 radiation, λ = 1.5406 Å |
| b = 8.282 (8) Å | T = 296 K |
| c = 5.071 (4) Å | flat sheet, 20 × 20 mm |
Data collection top
Bruker D8 Advance
diffractometer | Scan method: step |
| Specimen mounting: packed powder pellet | 2θmin = 8.0°, 2θmax = 140.422°, 2θstep = 0.017° |
| Data collection mode: reflection | |
Refinement top
| Rp = 0.048 | R(F2) = 0.04372 |
| Rwp = 0.063 | χ2 = 6.250 |
| Rexp = 0.025 | 7939 data points |
| RBragg = 0.047 | 81 parameters |
| R(F) = 0.049 | 0 restraints |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2) top| | x | y | z | Uiso*/Ueq | |
| Mg1 | 0.6095 | 0.1432 | 0.092 | 0.015* | |
| Mg2 | 0.0 | 0.0 | 0.5 | 0.015* | |
| P1 | 0.1996 | 0.1946 | 0.0355 | 0.015* | |
| O1 | 0.0589 | 0.1446 | 0.8188 | 0.015* | |
| O2 | 0.1262 | 0.1995 | 0.3036 | 0.015* | |
| O3 | 0.2592 | 0.3629 | 0.947 | 0.015* | |
| O4 | 0.3545 | 0.0759 | 0.0459 | 0.015* | |
Atomic displacement parameters (Å2) top| | U11 | U22 | U33 | U12 | U13 | U23 |
| ? | ? | ? | ? | ? | ? | ? |
Geometric parameters (Å, º) top
| Mg1—O1i | 2.1496 (14) | P1—O4 | 1.5310 (7) |
| Mg1—O2i | 1.9692 (11) | O1—Mg1vii | 2.1496 (14) |
| Mg1—O3i | 2.0613 (16) | O1—Mg2 | 2.0363 (12) |
| Mg1—O4 | 2.0128 (9) | O1—P1viii | 1.5350 (11) |
| Mg1—O4ii | 1.9706 (16) | O2—Mg1vii | 1.9692 (11) |
| Mg2—O1 | 2.0363 (12) | O2—Mg2 | 2.1849 (13) |
| Mg2—O1iii | 2.0363 (12) | O2—P1 | 1.5055 (11) |
| Mg2—O2 | 2.1849 (13) | O3—Mg1vii | 2.0613 (16) |
| Mg2—O2iii | 2.1849 (13) | O3—Mg2ix | 2.1532 (9) |
| Mg2—O3iv | 2.1532 (9) | O3—P1viii | 1.5419 (12) |
| Mg2—O3v | 2.1532 (9) | O4—Mg1 | 2.0128 (9) |
| P1—O1vi | 1.5350 (11) | O4—Mg1ii | 1.9706 (16) |
| P1—O2 | 1.5055 (11) | O4—P1 | 1.5310 (7) |
| P1—O3vi | 1.5419 (12) | | |
| | | |
| O1i—Mg1—O2i | 83.09 (6) | O2iii—Mg2—O3iv | 86.56 (5) |
| O1i—Mg1—O3i | 70.19 (3) | O2iii—Mg2—O3v | 93.44 (5) |
| O1x—Mg1—O4 | 94.70 (4) | O3iv—Mg2—O3v | 180.0 |
| O1x—Mg1—O4ii | 167.723 (8) | O1vi—P1—O2 | 111.75 (5) |
| O2i—Mg1—O3i | 127.20 (3) | O1vi—P1—O3vi | 103.84 (3) |
| O2x—Mg1—O4 | 102.03 (5) | O1vi—P1—O4 | 110.24 (4) |
| O2x—Mg1—O4ii | 109.14 (6) | O2—P1—O3vi | 111.91 (3) |
| O3x—Mg1—O4 | 124.19 (5) | O2—P1—O4 | 108.56 (4) |
| O3x—Mg1—O4ii | 101.87 (2) | O3vi—P1—O4 | 110.49 (5) |
| O4—Mg1—O4ii | 81.95 (3) | Mg1xi—O1—Mg2 | 95.37 (6) |
| O1—Mg2—O1iii | 180.0 | Mg1xii—O1—P1viii | 91.20 (4) |
| O1—Mg2—O2 | 80.69 (6) | Mg2—O1—P1viii | 146.12 (2) |
| O1—Mg2—O2iii | 99.31 (6) | Mg1xi—O2—Mg2 | 96.24 (6) |
| O1—Mg2—O3iv | 94.22 (6) | Mg1xii—O2—P1 | 137.36 (3) |
| O1—Mg2—O3v | 85.78 (6) | Mg2—O2—P1 | 126.38 (3) |
| O1iii—Mg2—O2 | 99.31 (6) | Mg1xi—O3—Mg2ix | 122.25 (4) |
| O1iii—Mg2—O2iii | 80.69 (6) | Mg1xii—O3—P1viii | 94.41 (2) |
| O1iii—Mg2—O3iv | 85.78 (6) | Mg2ix—O3—P1viii | 134.78 (3) |
| O1iii—Mg2—O3v | 94.22 (6) | Mg1—O4—Mg1ii | 98.05 (3) |
| O2—Mg2—O2iii | 179.9802 | Mg1—O4—P1 | 123.83 (4) |
| O2—Mg2—O3iv | 93.44 (5) | Mg1ii—O4—P1 | 134.78 (3) |
| O2—Mg2—O3v | 86.56 (5) | | |
| Symmetry codes: (i) x+1/2, −y+1/2, z−1/2; (ii) −x+1, −y, −z; (iii) −x, −y, −z+1; (iv) −x+1/2, y−1/2, −z+3/2; (v) x−1/2, −y+1/2, z−1/2; (vi) x, y, z−1; (vii) x−1/2, −y+1/2, z+1/2; (viii) x, y, z+1; (ix) −x+1/2, y+1/2, −z+3/2; (x) x+3/2, −y+3/2, z+1/2; (xi) x+1/2, −y+3/2, z+1/2; (xii) x−1/2, −y+1/2, z+3/2. |
Experimental details
| | (phase_1) | (phase_2) | (phase_3) |
| Crystal data |
| Chemical formula | Sr2.36Mg2.64P4O15 | Mg2P2O7 | Mg3P2O8 |
| Mr | 634.65 | 222.56 | 262.87 |
| Crystal system, space group | Orthorhombic, Cmcm | Monoclinic, P21/c | Monoclinic, P21/n |
| Temperature (K) | 296 | 296 | 296 |
| a, b, c (Å) | 14.27366 (9), 11.75678 (7), 7.19753 (4) | 6.9443 (4), 8.2861 (4), 9.0438 (5) | 7.594 (3), 8.282 (8), 5.071 (4) |
| α, β, γ (°) | 90, 90, 90 | 90, 113.816 (3), 90 | 90, 94.01 (6), 90 |
| V (Å3) | 1207.83 (1) | 476.08 (5) | 318.1 (4) |
| Z | 4 | 4 | 2 |
| Radiation type | Cu Kα1, λ = 1.5406 Å | Cu Kα1, λ = 1.5406 Å | Cu Kα1, λ = 1.5406 Å |
| Specimen shape, size (mm) | Flat sheet, 20 × 20 | Flat sheet, 20 × 20 | Flat sheet, 20 × 20 |
| |
| Data collection |
| Diffractometer | Bruker D8 Advance
diffractometer | Bruker D8 Advance
diffractometer | Bruker D8 Advance
diffractometer |
| Specimen mounting | Packed powder pellet | Packed powder pellet | Packed powder pellet |
| Data collection mode | Reflection | Reflection | Reflection |
| Scan method | Step | Step | Step |
| 2θ values (°) | 2θmin = 8.0 2θmax = 140.422 2θstep = 0.017 | 2θmin = 8.0 2θmax = 140.422 2θstep = 0.017 | 2θmin = 8.0 2θmax = 140.422 2θstep = 0.017 |
| |
| Refinement |
| R factors and goodness of fit | Rp = 0.048, Rwp = 0.063, Rexp = 0.025, RBragg = 0.047, R(F) = 0.028, R(F2) = 0.04372, χ2 = 6.250 | Rp = 0.048, Rwp = 0.063, Rexp = 0.025, RBragg = 0.047, R(F) = 0.047, R(F2) = 0.04372, χ2 = 6.250 | Rp = 0.048, Rwp = 0.063, Rexp = 0.025, RBragg = 0.047, R(F) = 0.049, R(F2) = 0.04372, χ2 = 6.250 |
| No. of data points | 7939 | 7939 | 7939 |
| No. of parameters | 81 | 81 | 81 |

 Subscribe to Acta Crystallographica Section C: Structural Chemistry
Subscribe to Acta Crystallographica Section C: Structural Chemistry
The full text of this article is available to subscribers to the journal.
If you have already registered and are using a computer listed in your registration details, please email
support@iucr.org for assistance.
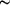 0.36), determined and refined from laboratory powder X-ray diffraction data, represents a new structure type. The title compound was synthesized by high-temperature solid-state reaction and it crystallizes in the orthorhombic space group Cmcm. It was earlier thought to be stoichiometric Sr2Mg3P4O15, but our structural study indicates the nonstoichiometric composition. The asymmetric unit contains one Sr (site symmetry ..m on special position 8g), one M (= Mg 64%/Sr 36%; site symmetry 2/m.. on special position 4b), one Mg (site symmetry 2.. on special position 8e), two P (site symmetry m.. on special position 8f and site symmetry ..m on special position 8g), and six O sites [two on general positions 16h, two on 8g, one on 8f and one on special position 4c (site symmetry m2m)]. The nonstoichiometry is due to the mixing of magnesium and strontium ions on the M site. The structure consists of three-dimensional networks of MgO4 and PO4 tetrahedra, and MO6 octahedra with the other strontium ions occupying the larger cavities surrounded by ten O atoms. All the polyhedra are connected by corner-sharing except the edge-sharing MO6 octahedra forming one-dimensional arrangements along [001].
0.36), determined and refined from laboratory powder X-ray diffraction data, represents a new structure type. The title compound was synthesized by high-temperature solid-state reaction and it crystallizes in the orthorhombic space group Cmcm. It was earlier thought to be stoichiometric Sr2Mg3P4O15, but our structural study indicates the nonstoichiometric composition. The asymmetric unit contains one Sr (site symmetry ..m on special position 8g), one M (= Mg 64%/Sr 36%; site symmetry 2/m.. on special position 4b), one Mg (site symmetry 2.. on special position 8e), two P (site symmetry m.. on special position 8f and site symmetry ..m on special position 8g), and six O sites [two on general positions 16h, two on 8g, one on 8f and one on special position 4c (site symmetry m2m)]. The nonstoichiometry is due to the mixing of magnesium and strontium ions on the M site. The structure consists of three-dimensional networks of MgO4 and PO4 tetrahedra, and MO6 octahedra with the other strontium ions occupying the larger cavities surrounded by ten O atoms. All the polyhedra are connected by corner-sharing except the edge-sharing MO6 octahedra forming one-dimensional arrangements along [001].
 Subscribe to Acta Crystallographica Section C: Structural Chemistry
Subscribe to Acta Crystallographica Section C: Structural Chemistry
 journal menu
journal menu








![[Figure 1]](http://journals.iucr.org/c/issues/2011/01/00/sq3270/sq3270fig1thm.gif)
![[Figure 2]](http://journals.iucr.org/c/issues/2011/01/00/sq3270/sq3270fig2thm.gif)
![[Figure 3]](http://journals.iucr.org/c/issues/2011/01/00/sq3270/sq3270fig3thm.gif)

 Subscribe to Acta Crystallographica Section C: Structural Chemistry
Subscribe to Acta Crystallographica Section C: Structural Chemistry



Sr2Mg3P4O15:Eu2+ was recently reported to show potential for application as a blue phosphor for a white LED under excitation of near-UV light (Ngee et al., 2009; Guo et al., 2010). The existence of a single phase in the system SrO–MgO–P2O5 was first confirmed over 40 years ago (Hoffman, 1968), but only un-indexed X-ray diffraction data were reported and its crystal structure has remained unknown. In the present study, during the course of crystal structure determination we have discovered that the compound previously known as Sr2Mg3P4O15 is in fact nonstoichiometric Sr2+xMg3-xP4O15 (x~0.36). Here we present its crystal structure, as determined and refined from laboratory powder X-ray diffraction data (Fig. 1).
Sr2+xMg3-xP4O15 crystallizes in a new structure type in terms of atomic ratios (6:1:2:15 for tetrahedral:octahedral:ten-coordinated metal:oxygen) and its polyhedral network is, to our knowledge, unique. The structure consists of (Mg2)O4, (P1)O4, and (P2)O4 tetrahedra, and MO6 octahedra, where M (≡ Mg1/Sr2) represents disordered magnesium (Mg1, 64%) and strontium (Sr2, 36%) ions (Fig. 2). The other strontium (Sr1) ions occupy the larger cavities surrounded by O atoms with tenfold coordination (Fig. 3). Each (Mg2)O4 tetrahedron is engaged in corner-sharing with two (P1)O4 and two (P2)O4 tetrahedra. (P1)O4 corner-shares with another (P1)O4 (to form a P2O7 group), two (Mg2)O4 and one MO6 polyhedra. (P2)O4 is connected to only two polyhedra: (Mg2)O4 and MO6. Each O6 atom in the other two corners of the P2 tetrahedron is bonded to two Sr1 ions, one in common to both O atoms. The MO6 octahedra form one-dimensional arrangements along [001], sharing edges. Each MO6 is also engaged in corner-sharing with two opposite-sided (P1)O4 tetrahedra along [010] and four (P2)O4 tetrahedra parallel to the (010) plane. The average M—O distance of 2.265 Å is very close to the expected value from the sum of M and O ionic radii (2.27Å) weighted by 64% Mg and 36% Sr (Shannon, 1976).
The empirical expression for bond valence, which has been widely adopted to estimate valences in inorganic solids (Brown, 2002), was used to check the Sr2+xMg3-xP4O15 (x~0.36) crystal structure. The bond-valence sums (Brown & Altermatt, 1985; Brese & O'Keeffe, 1991) calculated with the program VaList (Wills, 2010) for Sr1 (2.04 v.u.), (Mg1/Sr2) (2.30), Mg2 (2.11), P1 (5.06), P2 (4.86), O1 (2.09), O2 (2.12), O3 (2.00), O4 (2.12), O5 (2.20) and O6 (1.85) match the expected charges of the ions reasonably well. The higher valence sum for (Mg1/Sr2) resulted from the shorter Sr2—O bond distances (2.265 Å). All the other interatomic distances (Table 1) are within the expected ranges.
The nonstoichiometry model with more strontium and fewer magnesium ions also conformed to the synthesis results that magnesium phosphates were the impurity phases. Several trials to prepare a single phase Sr2+xMg3-xP4O15 (x~0.36) with the nonstoichiometric nominal composition under the same synthesis conditions were unsuccessful: the magnesium phosphate impurities disappeared but instead SrMgP2O7 (Tahiri et al., 2002) appeared as an impurity phase in the X-ray diffraction pattern. The relative atomic stoichiometries of Sr2.36Mg2.64P4O15 and SrMgP2O7 are quite similar and they seemed to compete with each other kinetically and/or thermodynamically in our synthetic condition. More careful work should be undertaken to determine a reproducible synthetic condition for the single phase, which is beyond the purpose of the present study.
The crystal structure of SrMgP2O7 is rather simple compared to Sr2.36Mg2.64P4O15. It consists of PO4 tetrahedra, MgO5 square-pyramids and eight-coordinated strontium ions occupying the larger cavities surrounded by O atoms. Each PO4 corner-shares with another PO4 [to form a P2O7 group, similar to (P1)O4 in Sr2.36Mg2.64P4O15] and three MgO5 polyhedra. All five corners of MgO5 are connected to PO4 tetrahedra. There is no MgO4 tetrahedron or octahedron in this structure, unlike Sr2.36Mg2.64P4O15.