
The 1:1 adduct of piperazine and ethane-1,2-diphosphonic acid is a salt [C
4H
12N
2]
2+·[C
2H
6O
6P
2]
2−, in which both ions lie across centres of inversion in space group
P2
1/
c. The anions are linked by a single type of O—H

O hydrogen bond [O

O, 2.562 (3) Å; H

O, 1.73 Å, O—H

O, 169°] into (6, 3) nets built from a single type of
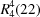
ring. The cations lie between these nets, linked to them by two types of N—H

O hydrogen bond [N

O, 2.635 (3) and 2.735 (3) Å; H

O 1.72 and 1.82 Å, N—H

O, 175 and 177°] such that the cations link adjacent sheets, thus forming a pillared-layer framework. The aquated adduct formed between trimethylenedipiperidine and ethane-1,2-diphosphonic acid is also a salt [C
13H
28N
2]
2+·[C
2H
6O
6P
2]
2−·2.8[H
2O], in which there are 12 different types of hydrogen bond, eight O—H

O and four N—H

O. The anions are linked into chains by pairs of O—H

O hydrogen bonds and these chains are linked by the water molecules into a continuous three-dimensional framework. Within the anion/water framework are large voids which contain pairs of cations, linked to the framework by N—H

O hydrogen bonds.
Supporting information
CCDC references: 159972; 159973
For both compounds, data collection: Kappa-CCD server software (Nonius, 1997); cell refinement: DENZO-SMN (Otwinowski & Minor, 1997); data reduction: DENZO-SMN (Otwinowski & Minor, 1997); program(s) used to solve structure: SHELXS97 (Sheldrick, 1997a); program(s) used to refine structure: NRCVAX96 and SHELXL97 (Sheldrick, 1997b); molecular graphics: NRCVAX96, ORTEP (Johnson, 1976), PLATON (Spek, 2000); software used to prepare material for publication: NRCVAX96, SHELXL97 and WORDPERFECT macro PRPKAPPA (Ferguson, 1999).
(18) Piperazine-ethane-1,2-diphosphonic acid (1/1)
top
Crystal data top
| C4H12N2·C2H6O6P2 | F(000) = 292 |
| Mr = 276.16 | Dx = 1.488 Mg m−3 |
| Monoclinic, P21/c | Mo Kα radiation, λ = 0.71073 Å |
| a = 7.3914 (6) Å | Cell parameters from 3237 reflections |
| b = 9.3343 (7) Å | θ = 2.9–27.4° |
| c = 9.3590 (6) Å | µ = 0.37 mm−1 |
| β = 107.291 (4)° | T = 150 K |
| V = 616.53 (8) Å3 | Needle, colourless |
| Z = 2 | 0.20 × 0.06 × 0.02 mm |
Data collection top
Kappa-CCD
diffractometer | 1401 independent reflections |
| Radiation source: fine-focus sealed X-ray tube | 947 reflections with I > 2σ(I) |
| Graphite monochromator | Rint = 0.057 |
| ϕ scans and ω scans with κ offsets | θmax = 27.4°, θmin = 2.9° |
Absorption correction: multi-scan
DENZO-SMN (Otwinowski & Minor, 1997) | h = 0→9 |
| Tmin = 0.930, Tmax = 0.993 | k = 0→12 |
| 3237 measured reflections | l = −12→11 |
Refinement top
| Refinement on F2 | Primary atom site location: structure-invariant direct methods |
| Least-squares matrix: full | Secondary atom site location: difference Fourier map |
| R[F2 > 2σ(F2)] = 0.059 | Hydrogen site location: inferred from neighbouring sites |
| wR(F2) = 0.123 | H-atom parameters constrained |
| S = 1.04 | w = 1/[σ2(Fo2) + (0.0312P)2 + 0.6688P]
where P = (Fo2 + 2Fc2)/3 |
| 1401 reflections | (Δ/σ)max < 0.001 |
| 74 parameters | Δρmax = 0.37 e Å−3 |
| 0 restraints | Δρmin = −0.43 e Å−3 |
Crystal data top
| C4H12N2·C2H6O6P2 | V = 616.53 (8) Å3 |
| Mr = 276.16 | Z = 2 |
| Monoclinic, P21/c | Mo Kα radiation |
| a = 7.3914 (6) Å | µ = 0.37 mm−1 |
| b = 9.3343 (7) Å | T = 150 K |
| c = 9.3590 (6) Å | 0.20 × 0.06 × 0.02 mm |
| β = 107.291 (4)° | |
Data collection top
Kappa-CCD
diffractometer | 1401 independent reflections |
Absorption correction: multi-scan
DENZO-SMN (Otwinowski & Minor, 1997) | 947 reflections with I > 2σ(I) |
| Tmin = 0.930, Tmax = 0.993 | Rint = 0.057 |
| 3237 measured reflections | |
Refinement top
| R[F2 > 2σ(F2)] = 0.059 | 0 restraints |
| wR(F2) = 0.123 | H-atom parameters constrained |
| S = 1.04 | Δρmax = 0.37 e Å−3 |
| 1401 reflections | Δρmin = −0.43 e Å−3 |
| 74 parameters | |
Special details top
Experimental. The program DENZO-SMN (Otwinowski & Minor, 1997) uses a scaling algorithm
(Fox & Holmes, 1966) which effectively corrects for absorption effects. High
redundancy data were used in the scaling program hence the 'multi-scan' code
word was used. No transmission coefficients are available from the program
(only scale factors for each frame). The scale factors in the experimental
table are calculated from the 'size' command in the SHELXL97 input
file. |
Geometry. Mean-plane data from the final SHELXL97 refinement run:- |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2) top| | x | y | z | Uiso*/Ueq | |
| P1 | 0.94421 (13) | 0.41065 (9) | 0.20303 (9) | 0.0164 (3) | |
| O1 | 0.7583 (3) | 0.3578 (2) | 0.2177 (2) | 0.0206 (6) | |
| O2 | 1.0375 (3) | 0.5304 (2) | 0.3082 (2) | 0.0205 (6) | |
| O3 | 1.0943 (3) | 0.2861 (2) | 0.2286 (3) | 0.0220 (6) | |
| C1 | 0.9100 (4) | 0.4705 (4) | 0.0138 (3) | 0.0204 (8) | |
| N1 | 0.6521 (4) | 0.4553 (3) | 0.4446 (3) | 0.0208 (7) | |
| C2 | 0.5123 (5) | 0.3499 (4) | 0.4674 (4) | 0.0219 (8) | |
| C3 | 0.5674 (5) | 0.5999 (4) | 0.4102 (4) | 0.0236 (8) | |
| H3 | 1.0379 | 0.2069 | 0.2118 | 0.033* | |
| H1A | 0.8626 | 0.3892 | −0.0551 | 0.024* | |
| H1B | 0.8115 | 0.5460 | −0.0102 | 0.024* | |
| H1C | 0.7540 | 0.4597 | 0.5296 | 0.025* | |
| H1D | 0.6954 | 0.4251 | 0.3670 | 0.025* | |
| H2A | 0.5746 | 0.2557 | 0.4949 | 0.026* | |
| H2B | 0.4081 | 0.3381 | 0.3731 | 0.026* | |
| H3A | 0.4652 | 0.5972 | 0.3139 | 0.028* | |
| H3B | 0.6653 | 0.6682 | 0.4000 | 0.028* | |
Atomic displacement parameters (Å2) top| | U11 | U22 | U33 | U12 | U13 | U23 |
| P1 | 0.0180 (5) | 0.0168 (5) | 0.0148 (4) | −0.0004 (4) | 0.0053 (3) | −0.0008 (4) |
| O1 | 0.0195 (13) | 0.0258 (13) | 0.0191 (12) | −0.0030 (11) | 0.0095 (10) | −0.0028 (10) |
| O2 | 0.0240 (14) | 0.0151 (12) | 0.0191 (11) | −0.0036 (10) | 0.0013 (10) | −0.0046 (10) |
| O3 | 0.0212 (14) | 0.0114 (12) | 0.0330 (13) | −0.0015 (10) | 0.0074 (11) | −0.0018 (11) |
| C1 | 0.0202 (19) | 0.025 (2) | 0.0157 (16) | −0.0028 (16) | 0.0047 (14) | 0.0001 (14) |
| N1 | 0.0199 (16) | 0.0273 (17) | 0.0162 (14) | 0.0013 (13) | 0.0069 (12) | −0.0021 (12) |
| C2 | 0.0227 (19) | 0.0210 (18) | 0.0219 (17) | 0.0010 (16) | 0.0064 (15) | 0.0019 (15) |
| C3 | 0.025 (2) | 0.026 (2) | 0.0214 (17) | −0.0006 (17) | 0.0095 (15) | 0.0038 (15) |
Geometric parameters (Å, º) top
| P1—O1 | 1.504 (2) | N1—C2 | 1.487 (4) |
| P1—O2 | 1.514 (2) | N1—H1C | 0.9200 |
| P1—O3 | 1.575 (2) | N1—H1D | 0.9200 |
| P1—C1 | 1.802 (3) | C2—C3ii | 1.510 (5) |
| O3—H3 | 0.8400 | C2—H2A | 0.9900 |
| C1—C1i | 1.531 (6) | C2—H2B | 0.9900 |
| C1—H1A | 0.9900 | C3—C2ii | 1.510 (5) |
| C1—H1B | 0.9900 | C3—H3A | 0.9900 |
| N1—C3 | 1.482 (4) | C3—H3B | 0.9900 |
| | | |
| O1—P1—O2 | 115.77 (13) | C3—N1—H1D | 109.3 |
| O1—P1—O3 | 111.42 (13) | C2—N1—H1D | 109.3 |
| O2—P1—O3 | 106.39 (13) | H1C—N1—H1D | 108.0 |
| O1—P1—C1 | 109.04 (13) | N1—C2—C3ii | 110.4 (3) |
| O2—P1—C1 | 108.31 (14) | N1—C2—H2A | 109.6 |
| O3—P1—C1 | 105.36 (15) | C3ii—C2—H2A | 109.6 |
| P1—O3—H3 | 109.5 | N1—C2—H2B | 109.6 |
| C1i—C1—P1 | 113.7 (3) | C3ii—C2—H2B | 109.6 |
| C1i—C1—H1A | 108.8 | H2A—C2—H2B | 108.1 |
| P1—C1—H1A | 108.8 | N1—C3—C2ii | 110.7 (3) |
| C1i—C1—H1B | 108.8 | N1—C3—H3A | 109.5 |
| P1—C1—H1B | 108.8 | C2ii—C3—H3A | 109.5 |
| H1A—C1—H1B | 107.7 | N1—C3—H3B | 109.5 |
| C3—N1—C2 | 111.5 (3) | C2ii—C3—H3B | 109.5 |
| C3—N1—H1C | 109.3 | H3A—C3—H3B | 108.1 |
| C2—N1—H1C | 109.3 | | |
| | | |
| O1—P1—C1—C1i | 178.9 (3) | C3—N1—C2—C3ii | −56.5 (4) |
| O2—P1—C1—C1i | 52.1 (4) | C2—N1—C3—C2ii | 56.7 (4) |
| O3—P1—C1—C1i | −61.4 (4) | | |
| Symmetry codes: (i) −x+2, −y+1, −z; (ii) −x+1, −y+1, −z+1. |
Hydrogen-bond geometry (Å, º) top
| D—H···A | D—H | H···A | D···A | D—H···A |
| O3—H3···O2iii | 0.84 | 1.73 | 2.562 (2) | 169 |
| N1—H1C···O2iv | 0.92 | 1.82 | 2.735 (3) | 177 |
| N1—H1D···O1 | 0.92 | 1.72 | 2.635 (3) | 175 |
| C2—H2A···O1v | 0.99 | 2.37 | 3.166 (4) | 136 |
| C2—H2B···O3vi | 0.99 | 2.36 | 3.285 (5) | 155 |
| Symmetry codes: (iii) −x+2, y−1/2, −z+1/2; (iv) −x+2, −y+1, −z+1; (v) x, −y+1/2, z+1/2; (vi) x−1, y, z. |
(17) 4,4'-trimethylenedipiperidine-ethane-1,2-diphosphonic acid-water (1/1/2.8).
top
Crystal data top
| (C13H28N2)·(C2H6O6P2)·2.8(H2O) | F(000) = 973 |
| Mr = 450.01 | Dx = 1.332 Mg m−3 |
| Monoclinic, P21/c | Mo Kα radiation, λ = 0.71073 Å |
| a = 6.1218 (2) Å | Cell parameters from 13672 reflections |
| b = 20.6215 (7) Å | θ = 3.0–30.1° |
| c = 18.1831 (7) Å | µ = 0.24 mm−1 |
| β = 99.942 (2)° | T = 120 K |
| V = 2260.98 (14) Å3 | Block, colourless |
| Z = 4 | 0.30 × 0.12 × 0.12 mm |
Data collection top
Kappa-CCD
diffractometer | 6599 independent reflections |
| Radiation source: fine-focus sealed X-ray tube | 4617 reflections with I > 2σ(I) |
| Graphite monochromator | Rint = 0.033 |
| ϕ scans and ω scans with κ offsets | θmax = 30.1°, θmin = 3.0° |
Absorption correction: multi-scan
DENZO-SMN (Otwinowski & Minor, 1997) | h = 0→8 |
| Tmin = 0.932, Tmax = 0.972 | k = −29→0 |
| 13672 measured reflections | l = −25→24 |
Refinement top
| Refinement on F2 | Primary atom site location: structure-invariant direct methods |
| Least-squares matrix: full | Secondary atom site location: difference Fourier map |
| R[F2 > 2σ(F2)] = 0.051 | Hydrogen site location: inferred from neighbouring sites |
| wR(F2) = 0.114 | H-atom parameters constrained |
| S = 1.02 | w = 1/[σ2(Fo2) + (0.0333P)2 + 1.181P]
where P = (Fo2 + 2Fc2)/3 |
| 6599 reflections | (Δ/σ)max = 0.001 |
| 284 parameters | Δρmax = 0.40 e Å−3 |
| 6 restraints | Δρmin = −0.39 e Å−3 |
Crystal data top
| (C13H28N2)·(C2H6O6P2)·2.8(H2O) | V = 2260.98 (14) Å3 |
| Mr = 450.01 | Z = 4 |
| Monoclinic, P21/c | Mo Kα radiation |
| a = 6.1218 (2) Å | µ = 0.24 mm−1 |
| b = 20.6215 (7) Å | T = 120 K |
| c = 18.1831 (7) Å | 0.30 × 0.12 × 0.12 mm |
| β = 99.942 (2)° | |
Data collection top
Kappa-CCD
diffractometer | 6599 independent reflections |
Absorption correction: multi-scan
DENZO-SMN (Otwinowski & Minor, 1997) | 4617 reflections with I > 2σ(I) |
| Tmin = 0.932, Tmax = 0.972 | Rint = 0.033 |
| 13672 measured reflections | |
Refinement top
| R[F2 > 2σ(F2)] = 0.051 | 6 restraints |
| wR(F2) = 0.114 | H-atom parameters constrained |
| S = 1.02 | Δρmax = 0.40 e Å−3 |
| 6599 reflections | Δρmin = −0.39 e Å−3 |
| 284 parameters | |
Special details top
Experimental. The program DENZO-SMN (Otwinowski & Minor, 1997) uses a scaling algorithm
(Fox & Holmes, 1966) which effectively corrects for absorption effects. High
redundancy data were used in the scaling program hence the 'multi-scan' code
word was used. No transmission coefficients are available from the program
(only scale factors for each frame). The scale factors in the experimental
table are calculated from the 'size' command in the SHELXL97 input
file. |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2) top| | x | y | z | Uiso*/Ueq | Occ. (<1) |
| N11 | 0.2398 (2) | 0.26517 (8) | 0.50540 (9) | 0.0190 (3) | |
| C12 | 0.1348 (3) | 0.33055 (10) | 0.50280 (12) | 0.0246 (4) | |
| C13 | 0.3078 (3) | 0.38363 (9) | 0.50220 (12) | 0.0231 (4) | |
| C14 | 0.4954 (3) | 0.37781 (9) | 0.56953 (11) | 0.0197 (4) | |
| C15 | 0.5957 (3) | 0.30998 (9) | 0.56861 (11) | 0.0198 (4) | |
| C16 | 0.4226 (3) | 0.25759 (10) | 0.57085 (11) | 0.0218 (4) | |
| C17 | 0.6671 (3) | 0.43166 (9) | 0.57012 (11) | 0.0227 (4) | |
| C18 | 0.8409 (3) | 0.43454 (9) | 0.64141 (11) | 0.0231 (4) | |
| C19 | 0.9987 (3) | 0.49186 (9) | 0.64262 (11) | 0.0225 (4) | |
| N21 | 1.4015 (2) | 0.56843 (7) | 0.84112 (9) | 0.0196 (3) | |
| C22 | 1.2669 (3) | 0.50973 (9) | 0.85170 (11) | 0.0213 (4) | |
| C23 | 1.0895 (3) | 0.50044 (10) | 0.78325 (11) | 0.0219 (4) | |
| C24 | 1.1834 (3) | 0.49508 (9) | 0.71087 (11) | 0.0198 (4) | |
| C25 | 1.3363 (3) | 0.55287 (9) | 0.70467 (11) | 0.0218 (4) | |
| C26 | 1.5105 (3) | 0.56218 (10) | 0.77452 (11) | 0.0227 (4) | |
| P1 | 0.51784 (7) | 0.18672 (2) | 0.36067 (3) | 0.01750 (11) | |
| P2 | 0.97414 (7) | 0.28310 (2) | 0.22866 (3) | 0.01652 (11) | |
| O11 | 0.5704 (2) | 0.13972 (7) | 0.42480 (8) | 0.0251 (3) | |
| O12 | 0.4089 (2) | 0.24934 (6) | 0.37945 (7) | 0.0201 (3) | |
| O13 | 0.3689 (2) | 0.15207 (6) | 0.29305 (8) | 0.0231 (3) | |
| O21 | 0.8935 (2) | 0.33129 (6) | 0.16740 (7) | 0.0220 (3) | |
| O22 | 1.0973 (2) | 0.22498 (6) | 0.20657 (7) | 0.0194 (3) | |
| O23 | 1.1228 (2) | 0.32169 (6) | 0.29441 (8) | 0.0221 (3) | |
| C1 | 0.7729 (3) | 0.20749 (9) | 0.32879 (10) | 0.0197 (4) | |
| C2 | 0.7328 (3) | 0.25730 (9) | 0.26563 (11) | 0.0189 (4) | |
| O3 | 0.3198 (2) | 0.08832 (8) | 0.52162 (8) | 0.0274 (3) | |
| O4 | −0.0510 (4) | 0.16833 (16) | 0.52524 (9) | 0.0251 (8) | 0.866 (11) |
| O41 | −0.122 (3) | 0.1948 (8) | 0.5260 (6) | 0.017 (4)* | 0.134 (11) |
| O5 | 0.1802 (7) | −0.02668 (15) | 0.5826 (3) | 0.0366 (14) | 0.585 (10) |
| O51 | 0.1100 (16) | −0.0376 (4) | 0.5439 (7) | 0.030 (3)* | 0.209 (11) |
| H11A | 0.2956 | 0.2586 | 0.4622 | 0.023* | |
| H11B | 0.1336 | 0.2340 | 0.5078 | 0.023* | |
| H12A | 0.0615 | 0.3362 | 0.5468 | 0.030* | |
| H12B | 0.0201 | 0.3341 | 0.4574 | 0.030* | |
| H13A | 0.2360 | 0.4266 | 0.5030 | 0.028* | |
| H13B | 0.3703 | 0.3805 | 0.4556 | 0.028* | |
| H14 | 0.4292 | 0.3820 | 0.6159 | 0.024* | |
| H15A | 0.6612 | 0.3047 | 0.5228 | 0.024* | |
| H15B | 0.7160 | 0.3050 | 0.6122 | 0.024* | |
| H16A | 0.4915 | 0.2143 | 0.5695 | 0.026* | |
| H16B | 0.3619 | 0.2611 | 0.6178 | 0.026* | |
| H17A | 0.5889 | 0.4738 | 0.5635 | 0.027* | |
| H17B | 0.7439 | 0.4254 | 0.5270 | 0.027* | |
| H18A | 0.7645 | 0.4375 | 0.6850 | 0.028* | |
| H18B | 0.9280 | 0.3938 | 0.6461 | 0.028* | |
| H19A | 0.9112 | 0.5324 | 0.6402 | 0.027* | |
| H19B | 1.0674 | 0.4900 | 0.5972 | 0.027* | |
| H21A | 1.3111 | 0.6044 | 0.8358 | 0.023* | |
| H21B | 1.5081 | 0.5744 | 0.8829 | 0.023* | |
| H22A | 1.1969 | 0.5152 | 0.8965 | 0.026* | |
| H22B | 1.3640 | 0.4710 | 0.8592 | 0.026* | |
| H23A | 0.9854 | 0.5375 | 0.7792 | 0.026* | |
| H23B | 1.0046 | 0.4606 | 0.7897 | 0.026* | |
| H24 | 1.2731 | 0.4544 | 0.7128 | 0.024* | |
| H25A | 1.4117 | 0.5463 | 0.6613 | 0.026* | |
| H25B | 1.2456 | 0.5928 | 0.6956 | 0.026* | |
| H26A | 1.6127 | 0.5246 | 0.7809 | 0.027* | |
| H26B | 1.5984 | 0.6017 | 0.7692 | 0.027* | |
| H13 | 0.2829 | 0.1793 | 0.2688 | 0.035* | |
| H23 | 1.2158 | 0.2964 | 0.3187 | 0.033* | |
| H1A | 0.8799 | 0.2254 | 0.3710 | 0.024* | |
| H1B | 0.8387 | 0.1678 | 0.3110 | 0.024* | |
| H2A | 0.6635 | 0.2961 | 0.2840 | 0.023* | |
| H2B | 0.6246 | 0.2388 | 0.2242 | 0.023* | |
| H31 | 0.197 (2) | 0.1081 (10) | 0.5193 (14) | 0.040* | |
| H32 | 0.380 (3) | 0.1032 (11) | 0.4864 (9) | 0.040* | |
| H41 | −0.173 (3) | 0.1595 (14) | 0.4967 (13) | 0.040* | 0.866 (11) |
| H42 | −0.066 (5) | 0.1721 (14) | 0.5706 (5) | 0.040* | 0.866 (11) |
| H51 | 0.206 (7) | −0.0030 (18) | 0.5468 (18) | 0.040* | 0.585 (10) |
| H52 | 0.244 (6) | −0.0617 (11) | 0.574 (2) | 0.040* | 0.585 (10) |
Atomic displacement parameters (Å2) top| | U11 | U22 | U33 | U12 | U13 | U23 |
| N11 | 0.0165 (7) | 0.0241 (8) | 0.0165 (8) | −0.0035 (6) | 0.0031 (6) | −0.0025 (6) |
| C12 | 0.0163 (8) | 0.0299 (11) | 0.0262 (11) | 0.0021 (8) | −0.0005 (8) | −0.0052 (9) |
| C13 | 0.0205 (9) | 0.0211 (10) | 0.0256 (11) | 0.0027 (7) | −0.0021 (8) | −0.0015 (8) |
| C14 | 0.0180 (8) | 0.0232 (10) | 0.0174 (9) | −0.0004 (7) | 0.0012 (7) | −0.0031 (8) |
| C15 | 0.0162 (8) | 0.0220 (10) | 0.0199 (9) | −0.0013 (7) | −0.0002 (7) | 0.0026 (8) |
| C16 | 0.0205 (9) | 0.0265 (10) | 0.0176 (10) | −0.0035 (8) | 0.0012 (7) | 0.0037 (8) |
| C17 | 0.0246 (9) | 0.0191 (9) | 0.0224 (10) | 0.0012 (8) | −0.0019 (8) | −0.0021 (8) |
| C18 | 0.0238 (9) | 0.0224 (10) | 0.0212 (10) | −0.0014 (8) | −0.0010 (8) | −0.0020 (8) |
| C19 | 0.0250 (9) | 0.0207 (10) | 0.0206 (10) | −0.0024 (8) | 0.0007 (8) | −0.0008 (8) |
| N21 | 0.0160 (7) | 0.0205 (8) | 0.0209 (8) | 0.0017 (6) | −0.0005 (6) | −0.0012 (7) |
| C22 | 0.0210 (9) | 0.0227 (10) | 0.0198 (10) | −0.0022 (7) | 0.0023 (7) | 0.0006 (8) |
| C23 | 0.0156 (8) | 0.0268 (10) | 0.0223 (10) | −0.0027 (7) | 0.0005 (7) | −0.0003 (8) |
| C24 | 0.0184 (8) | 0.0190 (9) | 0.0213 (10) | 0.0002 (7) | 0.0014 (7) | −0.0023 (8) |
| C25 | 0.0227 (9) | 0.0216 (10) | 0.0218 (10) | −0.0042 (8) | 0.0064 (8) | −0.0034 (8) |
| C26 | 0.0173 (8) | 0.0238 (10) | 0.0277 (11) | −0.0032 (7) | 0.0063 (8) | −0.0033 (8) |
| P1 | 0.0165 (2) | 0.0196 (2) | 0.0159 (2) | 0.00030 (18) | 0.00156 (17) | 0.00276 (19) |
| P2 | 0.0166 (2) | 0.0180 (2) | 0.0150 (2) | 0.00223 (18) | 0.00275 (17) | 0.00145 (19) |
| O11 | 0.0200 (6) | 0.0305 (8) | 0.0240 (8) | −0.0005 (6) | 0.0012 (6) | 0.0115 (6) |
| O12 | 0.0204 (6) | 0.0237 (7) | 0.0162 (7) | 0.0024 (5) | 0.0031 (5) | −0.0007 (5) |
| O13 | 0.0244 (7) | 0.0176 (7) | 0.0242 (8) | 0.0010 (5) | −0.0047 (6) | −0.0003 (6) |
| O21 | 0.0240 (6) | 0.0241 (7) | 0.0185 (7) | 0.0069 (5) | 0.0059 (5) | 0.0053 (5) |
| O22 | 0.0201 (6) | 0.0208 (7) | 0.0171 (7) | 0.0040 (5) | 0.0030 (5) | −0.0002 (5) |
| O23 | 0.0234 (7) | 0.0196 (7) | 0.0216 (7) | 0.0017 (5) | −0.0013 (6) | −0.0003 (6) |
| C1 | 0.0186 (8) | 0.0231 (10) | 0.0174 (9) | 0.0023 (7) | 0.0025 (7) | 0.0023 (8) |
| C2 | 0.0159 (8) | 0.0224 (10) | 0.0179 (9) | 0.0014 (7) | 0.0018 (7) | 0.0025 (8) |
| O3 | 0.0221 (7) | 0.0372 (9) | 0.0227 (8) | 0.0027 (6) | 0.0033 (6) | 0.0068 (7) |
| O4 | 0.0207 (11) | 0.0370 (17) | 0.0174 (9) | −0.0077 (11) | 0.0026 (7) | −0.0007 (8) |
| O5 | 0.040 (2) | 0.0316 (18) | 0.043 (3) | 0.0086 (13) | 0.0215 (19) | 0.0061 (15) |
Geometric parameters (Å, º) top
| N11—C12 | 1.491 (2) | C23—H23B | 0.9900 |
| N11—C16 | 1.494 (2) | C24—C25 | 1.532 (3) |
| N11—H11A | 0.9200 | C24—H24 | 1.0000 |
| N11—H11B | 0.9200 | C25—C26 | 1.523 (3) |
| C12—C13 | 1.524 (3) | C25—H25A | 0.9900 |
| C12—H12A | 0.9900 | C25—H25B | 0.9900 |
| C12—H12B | 0.9900 | C26—H26A | 0.9900 |
| C13—C14 | 1.532 (2) | C26—H26B | 0.9900 |
| C13—H13A | 0.9900 | P1—O11 | 1.5076 (14) |
| C13—H13B | 0.9900 | P1—O12 | 1.5189 (14) |
| C14—C17 | 1.528 (3) | P1—O13 | 1.5707 (13) |
| C14—C15 | 1.529 (3) | P1—C1 | 1.8086 (19) |
| C14—H14 | 1.0000 | P2—O22 | 1.5071 (13) |
| C15—C16 | 1.519 (2) | P2—O21 | 1.5105 (13) |
| C15—H15A | 0.9900 | P2—O23 | 1.5860 (13) |
| C15—H15B | 0.9900 | P2—C2 | 1.8055 (18) |
| C16—H16A | 0.9900 | O13—H13 | 0.8400 |
| C16—H16B | 0.9900 | O23—H23 | 0.8400 |
| C17—C18 | 1.530 (3) | C1—C2 | 1.529 (3) |
| C17—H17A | 0.9900 | C1—H1A | 0.9900 |
| C17—H17B | 0.9900 | C1—H1B | 0.9900 |
| C18—C19 | 1.524 (3) | C2—H2A | 0.9900 |
| C18—H18A | 0.9900 | C2—H2B | 0.9900 |
| C18—H18B | 0.9900 | O3—H31 | 0.85 (2) |
| C19—C24 | 1.529 (2) | O3—H32 | 0.85 (2) |
| C19—H19A | 0.9900 | O4—O41 | 0.701 (18) |
| C19—H19B | 0.9900 | O4—H41 | 0.850 (3) |
| N21—C26 | 1.486 (2) | O4—H42 | 0.850 (3) |
| N21—C22 | 1.495 (2) | O41—H41 | 0.92 (2) |
| N21—H21A | 0.9200 | O41—H42 | 0.95 (2) |
| N21—H21B | 0.9200 | O5—O51 | 0.791 (10) |
| C22—C23 | 1.516 (3) | O5—H51 | 0.850 (3) |
| C22—H22A | 0.9900 | O5—H52 | 0.850 (3) |
| C22—H22B | 0.9900 | O51—H51 | 0.92 (4) |
| C23—C24 | 1.528 (3) | O51—H52 | 1.03 (3) |
| C23—H23A | 0.9900 | | |
| | | |
| C12—N11—C16 | 112.30 (15) | H22A—C22—H22B | 108.3 |
| C12—N11—H11A | 109.1 | C22—C23—C24 | 113.18 (15) |
| C16—N11—H11A | 109.1 | C22—C23—H23A | 108.9 |
| C12—N11—H11B | 109.1 | C24—C23—H23A | 108.9 |
| C16—N11—H11B | 109.1 | C22—C23—H23B | 108.9 |
| H11A—N11—H11B | 107.9 | C24—C23—H23B | 108.9 |
| N11—C12—C13 | 110.69 (15) | H23A—C23—H23B | 107.8 |
| N11—C12—H12A | 109.5 | C23—C24—C19 | 111.52 (15) |
| C13—C12—H12A | 109.5 | C23—C24—C25 | 109.42 (15) |
| N11—C12—H12B | 109.5 | C19—C24—C25 | 110.47 (16) |
| C13—C12—H12B | 109.5 | C23—C24—H24 | 108.5 |
| H12A—C12—H12B | 108.1 | C19—C24—H24 | 108.5 |
| C12—C13—C14 | 111.34 (16) | C25—C24—H24 | 108.5 |
| C12—C13—H13A | 109.4 | C26—C25—C24 | 112.68 (16) |
| C14—C13—H13A | 109.4 | C26—C25—H25A | 109.1 |
| C12—C13—H13B | 109.4 | C24—C25—H25A | 109.1 |
| C14—C13—H13B | 109.4 | C26—C25—H25B | 109.1 |
| H13A—C13—H13B | 108.0 | C24—C25—H25B | 109.1 |
| C17—C14—C15 | 112.84 (15) | H25A—C25—H25B | 107.8 |
| C17—C14—C13 | 111.63 (16) | N21—C26—C25 | 110.04 (15) |
| C15—C14—C13 | 108.05 (15) | N21—C26—H26A | 109.7 |
| C17—C14—H14 | 108.1 | C25—C26—H26A | 109.7 |
| C15—C14—H14 | 108.1 | N21—C26—H26B | 109.7 |
| C13—C14—H14 | 108.1 | C25—C26—H26B | 109.7 |
| C16—C15—C14 | 111.55 (15) | H26A—C26—H26B | 108.2 |
| C16—C15—H15A | 109.3 | O11—P1—O12 | 114.59 (8) |
| C14—C15—H15A | 109.3 | O11—P1—O13 | 109.30 (8) |
| C16—C15—H15B | 109.3 | O12—P1—O13 | 110.17 (7) |
| C14—C15—H15B | 109.3 | O11—P1—C1 | 108.62 (8) |
| H15A—C15—H15B | 108.0 | O12—P1—C1 | 107.92 (8) |
| N11—C16—C15 | 109.52 (15) | O13—P1—C1 | 105.87 (8) |
| N11—C16—H16A | 109.8 | O22—P2—O21 | 116.20 (8) |
| C15—C16—H16A | 109.8 | O22—P2—O23 | 110.58 (7) |
| N11—C16—H16B | 109.8 | O21—P2—O23 | 107.33 (8) |
| C15—C16—H16B | 109.8 | O22—P2—C2 | 110.12 (8) |
| H16A—C16—H16B | 108.2 | O21—P2—C2 | 106.51 (8) |
| C14—C17—C18 | 114.29 (16) | O23—P2—C2 | 105.50 (8) |
| C14—C17—H17A | 108.7 | P1—O13—H13 | 109.5 |
| C18—C17—H17A | 108.7 | P2—O23—H23 | 109.5 |
| C14—C17—H17B | 108.7 | C2—C1—P1 | 111.37 (12) |
| C18—C17—H17B | 108.7 | C2—C1—H1A | 109.4 |
| H17A—C17—H17B | 107.6 | P1—C1—H1A | 109.4 |
| C19—C18—C17 | 112.88 (16) | C2—C1—H1B | 109.4 |
| C19—C18—H18A | 109.0 | P1—C1—H1B | 109.4 |
| C17—C18—H18A | 109.0 | H1A—C1—H1B | 108.0 |
| C19—C18—H18B | 109.0 | C1—C2—P2 | 116.24 (12) |
| C17—C18—H18B | 109.0 | C1—C2—H2A | 108.2 |
| H18A—C18—H18B | 107.8 | P2—C2—H2A | 108.2 |
| C18—C19—C24 | 115.05 (16) | C1—C2—H2B | 108.2 |
| C18—C19—H19A | 108.5 | P2—C2—H2B | 108.2 |
| C24—C19—H19A | 108.5 | H2A—C2—H2B | 107.4 |
| C18—C19—H19B | 108.5 | H31—O3—H32 | 107 (2) |
| C24—C19—H19B | 108.5 | O41—O4—H41 | 72 (2) |
| H19A—C19—H19B | 107.5 | O41—O4—H42 | 75 (2) |
| C26—N21—C22 | 111.69 (15) | H41—O4—H42 | 112 (3) |
| C26—N21—H21A | 109.3 | O4—O41—H41 | 61.3 (16) |
| C22—N21—H21A | 109.3 | O4—O41—H42 | 59.8 (15) |
| C26—N21—H21B | 109.3 | H41—O41—H42 | 98 (3) |
| C22—N21—H21B | 109.3 | O51—O5—H51 | 68 (3) |
| H21A—N21—H21B | 107.9 | O51—O5—H52 | 78 (3) |
| N21—C22—C23 | 109.22 (15) | H51—O5—H52 | 101 (4) |
| N21—C22—H22A | 109.8 | O5—O51—H51 | 58.9 (17) |
| C23—C22—H22A | 109.8 | O5—O51—H52 | 53.8 (16) |
| N21—C22—H22B | 109.8 | H51—O51—H52 | 84 (3) |
| C23—C22—H22B | 109.8 | | |
| | | |
| C16—N11—C12—C13 | 56.4 (2) | C22—C23—C24—C25 | −52.4 (2) |
| N11—C12—C13—C14 | −56.2 (2) | C18—C19—C24—C23 | −60.2 (2) |
| C12—C13—C14—C17 | −178.88 (16) | C18—C19—C24—C25 | 177.84 (16) |
| C12—C13—C14—C15 | 56.5 (2) | C23—C24—C25—C26 | 51.4 (2) |
| C17—C14—C15—C16 | 177.95 (16) | C19—C24—C25—C26 | 174.59 (16) |
| C13—C14—C15—C16 | −58.1 (2) | C22—N21—C26—C25 | 59.5 (2) |
| C12—N11—C16—C15 | −57.2 (2) | C24—C25—C26—N21 | −55.5 (2) |
| C14—C15—C16—N11 | 58.6 (2) | O11—P1—C1—C2 | −177.70 (13) |
| C15—C14—C17—C18 | −66.4 (2) | O12—P1—C1—C2 | −52.92 (15) |
| C13—C14—C17—C18 | 171.71 (16) | O13—P1—C1—C2 | 65.02 (15) |
| C14—C17—C18—C19 | −175.63 (16) | P1—C1—C2—P2 | 179.53 (10) |
| C17—C18—C19—C24 | −176.98 (17) | O22—P2—C2—C1 | 52.81 (16) |
| C26—N21—C22—C23 | −59.66 (19) | O21—P2—C2—C1 | 179.60 (14) |
| N21—C22—C23—C24 | 56.5 (2) | O23—P2—C2—C1 | −66.54 (16) |
| C22—C23—C24—C19 | −174.92 (16) | | |
Hydrogen-bond geometry (Å, º) top
| D—H···A | D—H | H···A | D···A | D—H···A |
| O13—H13···O22i | 0.84 | 1.74 | 2.568 (2) | 170 |
| O23—H23···O12ii | 0.84 | 1.76 | 2.598 (2) | 173 |
| O3—H31···O4 | 0.85 (2) | 1.98 (2) | 2.816 (3) | 168 (2) |
| O3—H32···O11 | 0.85 (2) | 1.91 (2) | 2.740 (2) | 167 (2) |
| O4—H41···O11i | 0.85 (2) | 1.91 (2) | 2.756 (3) | 174 (2) |
| O4—H42···O21iii | 0.85 (2) | 1.82 (2) | 2.665 (2) | 172 (3) |
| O5—H51···O3 | 0.85 (4) | 2.09 (4) | 2.813 (4) | 143 (3) |
| O5—H52···O11iv | 0.85 (3) | 1.97 (3) | 2.802 (4) | 168 (3) |
| N11—H11A···O12 | 0.92 | 1.77 | 2.691 (2) | 177 |
| N11—H11B···O4 | 0.92 | 1.83 | 2.740 (3) | 172 |
| N21—H21A···O21v | 0.92 | 1.82 | 2.732 (2) | 172 |
| N21—H21B···O3vi | 0.92 | 1.89 | 2.800 (3) | 169 |
| Symmetry codes: (i) x−1, y, z; (ii) x+1, y, z; (iii) x−1, −y+1/2, z+1/2; (iv) −x+1, −y, −z+1; (v) −x+2, −y+1, −z+1; (vi) −x+2, y+1/2, −z+3/2. |
Experimental details
| | (18) | (17) |
| Crystal data |
| Chemical formula | C4H12N2·C2H6O6P2 | (C13H28N2)·(C2H6O6P2)·2.8(H2O) |
| Mr | 276.16 | 450.01 |
| Crystal system, space group | Monoclinic, P21/c | Monoclinic, P21/c |
| Temperature (K) | 150 | 120 |
| a, b, c (Å) | 7.3914 (6), 9.3343 (7), 9.3590 (6) | 6.1218 (2), 20.6215 (7), 18.1831 (7) |
| β (°) | 107.291 (4) | 99.942 (2) |
| V (Å3) | 616.53 (8) | 2260.98 (14) |
| Z | 2 | 4 |
| Radiation type | Mo Kα | Mo Kα |
| µ (mm−1) | 0.37 | 0.24 |
| Crystal size (mm) | 0.20 × 0.06 × 0.02 | 0.30 × 0.12 × 0.12 |
| |
| Data collection |
| Diffractometer | Kappa-CCD
diffractometer | Kappa-CCD
diffractometer |
| Absorption correction | Multi-scan
DENZO-SMN (Otwinowski & Minor, 1997) | Multi-scan
DENZO-SMN (Otwinowski & Minor, 1997) |
| Tmin, Tmax | 0.930, 0.993 | 0.932, 0.972 |
No. of measured, independent and
observed [I > 2σ(I)] reflections | 3237, 1401, 947 | 13672, 6599, 4617 |
| Rint | 0.057 | 0.033 |
| (sin θ/λ)max (Å−1) | 0.647 | 0.705 |
| |
| Refinement |
| R[F2 > 2σ(F2)], wR(F2), S | 0.059, 0.123, 1.04 | 0.051, 0.114, 1.02 |
| No. of reflections | 1401 | 6599 |
| No. of parameters | 74 | 284 |
| No. of restraints | 0 | 6 |
| H-atom treatment | H-atom parameters constrained | H-atom parameters constrained |
| Δρmax, Δρmin (e Å−3) | 0.37, −0.43 | 0.40, −0.39 |
Hydrogen-bond geometry (Å, º) for (18) top
| D—H···A | D—H | H···A | D···A | D—H···A |
| O3—H3···O2i | 0.84 | 1.73 | 2.562 (2) | 169 |
| N1—H1C···O2ii | 0.92 | 1.82 | 2.735 (3) | 177 |
| N1—H1D···O1 | 0.92 | 1.72 | 2.635 (3) | 175 |
| C2—H2A···O1iii | 0.99 | 2.37 | 3.166 (4) | 136 |
| C2—H2B···O3iv | 0.99 | 2.36 | 3.285 (5) | 155 |
| Symmetry codes: (i) −x+2, y−1/2, −z+1/2; (ii) −x+2, −y+1, −z+1; (iii) x, −y+1/2, z+1/2; (iv) x−1, y, z. |
Hydrogen-bond geometry (Å, º) for (17) top
| D—H···A | D—H | H···A | D···A | D—H···A |
| O13—H13···O22i | 0.84 | 1.74 | 2.568 (2) | 170 |
| O23—H23···O12ii | 0.84 | 1.76 | 2.598 (2) | 173 |
| O3—H31···O4 | 0.85 (2) | 1.98 (2) | 2.816 (3) | 168 (2) |
| O3—H32···O11 | 0.85 (2) | 1.91 (2) | 2.740 (2) | 167 (2) |
| O4—H41···O11i | 0.85 (2) | 1.91 (2) | 2.756 (3) | 174 (2) |
| O4—H42···O21iii | 0.85 (2) | 1.82 (2) | 2.665 (2) | 172 (3) |
| O5—H51···O3 | 0.85 (4) | 2.09 (4) | 2.813 (4) | 143 (3) |
| O5—H52···O11iv | 0.85 (3) | 1.97 (3) | 2.802 (4) | 168 (3) |
| N11—H11A···O12 | 0.92 | 1.77 | 2.691 (2) | 177 |
| N11—H11B···O4 | 0.92 | 1.83 | 2.740 (3) | 172 |
| N21—H21A···O21v | 0.92 | 1.82 | 2.732 (2) | 172 |
| N21—H21B···O3vi | 0.92 | 1.89 | 2.800 (3) | 169 |
| Symmetry codes: (i) x−1, y, z; (ii) x+1, y, z; (iii) x−1, −y+1/2, z+1/2; (iv) −x+1, −y, −z+1; (v) −x+2, −y+1, −z+1; (vi) −x+2, y+1/2, −z+3/2. |
 O hydrogen bond [O
O hydrogen bond [O O, 2.562 (3) Å; H
O, 2.562 (3) Å; H O, 1.73 Å, O—H
O, 1.73 Å, O—H O, 169°] into (6, 3) nets built from a single type of
O, 169°] into (6, 3) nets built from a single type of  O hydrogen bond [N
O hydrogen bond [N O, 2.635 (3) and 2.735 (3) Å; H
O, 2.635 (3) and 2.735 (3) Å; H O 1.72 and 1.82 Å, N—H
O 1.72 and 1.82 Å, N—H O, 175 and 177°] such that the cations link adjacent sheets, thus forming a pillared-layer framework. The aquated adduct formed between trimethylenedipiperidine and ethane-1,2-diphosphonic acid is also a salt [C13H28N2]2+·[C2H6O6P2]2−·2.8[H2O], in which there are 12 different types of hydrogen bond, eight O—H
O, 175 and 177°] such that the cations link adjacent sheets, thus forming a pillared-layer framework. The aquated adduct formed between trimethylenedipiperidine and ethane-1,2-diphosphonic acid is also a salt [C13H28N2]2+·[C2H6O6P2]2−·2.8[H2O], in which there are 12 different types of hydrogen bond, eight O—H O and four N—H
O and four N—H O. The anions are linked into chains by pairs of O—H
O. The anions are linked into chains by pairs of O—H O hydrogen bonds and these chains are linked by the water molecules into a continuous three-dimensional framework. Within the anion/water framework are large voids which contain pairs of cations, linked to the framework by N—H
O hydrogen bonds and these chains are linked by the water molecules into a continuous three-dimensional framework. Within the anion/water framework are large voids which contain pairs of cations, linked to the framework by N—H O hydrogen bonds.
O hydrogen bonds.
 journal menu
journal menu








![[Figure 1]](http://journals.iucr.org/b/issues/2001/01/00/na0111/na0111fig1thm.gif)
![[Figure 2]](http://journals.iucr.org/b/issues/2001/01/00/na0111/na0111fig2thm.gif)
![[Figure 3]](http://journals.iucr.org/b/issues/2001/01/00/na0111/na0111fig3thm.gif)
![[Figure 4]](http://journals.iucr.org/b/issues/2001/01/00/na0111/na0111fig4thm.gif)
![[Figure 5]](http://journals.iucr.org/b/issues/2001/01/00/na0111/na0111fig5thm.gif)
![[Figure 6]](http://journals.iucr.org/b/issues/2001/01/00/na0111/na0111fig6thm.gif)
![[Figure 7]](http://journals.iucr.org/b/issues/2001/01/00/na0111/na0111fig7thm.gif)




In full text version