The crystal structures of pure L-carvone [(R)-(-)-2-methyl-5-(1-methylethenyl)-2-cyclohexen-1-one, C10H14O] and the equimolar mixture DL-carvone (RS) have been determined by Patterson-search methods at low resolution from laboratory X-ray powder diffraction data (218 K). Crystal data: (L) a = 6.8576 (3), b = 6.8831 (5), c = 19.988 (2) Å, P212121 space group, Z = 4; (DL) a = 6.9744 (3), b = 6.8094 (6), c = 20.038 (7) Å, Pcmn space group, Z = 4. The L-carvone structure has been refined by the Rietveld method as a rigid body, allowing the rotation of the isopropenyl group (R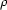 , = 0.030 and Rwp = 0.043). Although the structure of DL-carvone could be unambiguously established, the Rietveld refinement was not possible due to the existence of preferred orientation in the sample and the difficulty in modelling the disorder. The molecular packing is essentialy the same for both compounds and can be explained as a stacking of two different molecular layers in the [001] direction. In each layer the molecules are placed with their long axis perpendicular to the layer plane, in a head-to-tail manner. The great similarity between the molecular shapes of L and D enantiomers favours the positional disorder in DL-carvone. This result confirms the mixed crystal formation for the chiral carvone system as proposed in recent thermodynamic studies. The DL-carvone crystal must be considered as a pseudo-racemate, since both enantiomers are randomly distributed over all the lattice sites.
, = 0.030 and Rwp = 0.043). Although the structure of DL-carvone could be unambiguously established, the Rietveld refinement was not possible due to the existence of preferred orientation in the sample and the difficulty in modelling the disorder. The molecular packing is essentialy the same for both compounds and can be explained as a stacking of two different molecular layers in the [001] direction. In each layer the molecules are placed with their long axis perpendicular to the layer plane, in a head-to-tail manner. The great similarity between the molecular shapes of L and D enantiomers favours the positional disorder in DL-carvone. This result confirms the mixed crystal formation for the chiral carvone system as proposed in recent thermodynamic studies. The DL-carvone crystal must be considered as a pseudo-racemate, since both enantiomers are randomly distributed over all the lattice sites.

 journal menu
journal menu











