
Tris(ethylenediamine)zinc(II) sulfate, [Zn(C
2H
8N
2)
3]SO
4, (I), undergoes a reversible solid-solid phase transition during cooling, accompanied by a lowering of the symmetry from high-trigonal
P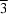
1
c to low-trigonal
P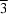
and by merohedral twinning. The molecular symmetries of the cation and anion change from 32 (
D3) to 3 (
C3). This lower symmetry allows an ordered sulfate anion and generates in the complex cation two independent N atoms with significantly different geometries. The twinning is the same as in the corresponding Ni complex [Jameson
et al. (1982).
Acta Cryst. B
38, 3016-3020]. The low-temperature phase of tris(ethylenediamine)copper(II) sulfate, [Cu(C
2H
8N
2)
3]SO
4, (II), has only triclinic symmetry and the unit-cell volume is doubled with respect to the room-temperature structure in
P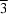
1
c. (II) was refined as a nonmerohedral twin with five twin domains. The asymmetric unit contains two independent formula units, and all cations and anions are located on general positions with 1 (
C1) symmetry. Both molecules of the Cu complex are in elongated octahedral geometries because of the Jahn-Teller effect. This is in contrast to an earlier publication, which describes the complex as a compressed octahedron [Bertini
et al. (1979).
J. Chem. Soc. Dalton Trans. pp. 1409-1414].
Supporting information
CCDC references: 804110; 804111
Compounds (I) and (II) were prepared by slowly adding an excess of
ethylenediamine at room temperature to a solution of the corresponding metal
salt in water. Single crystals were obtained by evaporating the solutions at
room temperature.
(I) was measured with a detector distance of 40 mm, a scan angle of 1° and an
exposure time of 15 s per frame. 652 frames were collected. The intensity data
of (I) were obtained using a single orientation matrix. For the refinement a
TWIN and a BASF instruction were included. All H atoms were located in
difference Fourier maps and refined freely with isotropic displacement
parameters.
(II) was measured with a detector distance of 50 mm, a scan angle of 0.5° and
an exposure time of 20 s per frame. 1615 frames were collected. The intensity
data of (II) were obtained using five orientation matrices. Only the single,
non-overlapping reflections of the major domain and the overlapping
reflections with the major domain were taken into account. These data were
merged prior to the refinement using the TWINABS software (Sheldrick,
2008a) resulting in an HKLF-5 type of reflection file
(Herbst-Irmer &
Sheldrick, 1998). H atoms were included in calculated positions and
refined
using a riding model with C—H = 0.99 and N—H = 0.92 Å and with
Uiso(H) = 1.2Uiso of the bearing C or N atom.
For both compounds, data collection: COLLECT (Nonius, 1999); cell refinement: PEAKREF (Schreurs, 2005). Data reduction: EVAL15 (Schreurs et al., 2010), SADABS (Sheldrick, 2008a) for (I); EVAL14 (Duisenberg et al., 2003), TWINABS (Sheldrick, 2008a) for (II). For both compounds, program(s) used to solve structure: SHELXS97 (Sheldrick, 2008b); program(s) used to refine structure: SHELXL97 (Sheldrick, 2008b); molecular graphics: PLATON (Spek, 2009); software used to prepare material for publication: manual edit of SHELXL cif file.
Crystal data top
| [Zn(C2H8N2)3]2+·SO42− | Dx = 1.711 Mg m−3 |
| Mr = 341.74 | Mo Kα radiation, λ = 0.71073 Å |
| Trigonal, P3 | Cell parameters from 13054 reflections |
| Hall symbol: -P 3 | θ = 2.1–35.0° |
| a = 8.89410 (11) Å | µ = 2.03 mm−1 |
| c = 9.68482 (13) Å | T = 110 K |
| V = 663.48 (2) Å3 | Needle, colourless |
| Z = 2 | 0.30 × 0.09 × 0.09 mm |
| F(000) = 360 | |
Data collection top
Nonius KappaCCD
diffractometer | 1355 independent reflections |
| Radiation source: rotating anode | 1338 reflections with I > 2σ(I) |
| Graphite monochromator | Rint = 0.028 |
| ϕ and ω scans | θmax = 30.5°, θmin = 2.1° |
Absorption correction: analytical
(SADABS; Sheldrick, 2008a) | h = −12→12 |
| Tmin = 0.612, Tmax = 0.872 | k = −12→12 |
| 11327 measured reflections | l = −13→13 |
Refinement top
| Refinement on F2 | Primary atom site location: structure-invariant direct methods |
| Least-squares matrix: full | Secondary atom site location: difference Fourier map |
| R[F2 > 2σ(F2)] = 0.015 | Hydrogen site location: difference Fourier map |
| wR(F2) = 0.038 | H atoms treated by a mixture of independent and constrained refinement |
| S = 1.06 | w = 1/[σ2(Fo2) + (0.0207P)2 + 0.1294P]
where P = (Fo2 + 2Fc2)/3 |
| 1355 reflections | (Δ/σ)max < 0.001 |
| 72 parameters | Δρmax = 0.48 e Å−3 |
| 0 restraints | Δρmin = −0.26 e Å−3 |
Crystal data top
| [Zn(C2H8N2)3]2+·SO42− | Z = 2 |
| Mr = 341.74 | Mo Kα radiation |
| Trigonal, P3 | µ = 2.03 mm−1 |
| a = 8.89410 (11) Å | T = 110 K |
| c = 9.68482 (13) Å | 0.30 × 0.09 × 0.09 mm |
| V = 663.48 (2) Å3 | |
Data collection top
Nonius KappaCCD
diffractometer | 1355 independent reflections |
Absorption correction: analytical
(SADABS; Sheldrick, 2008a) | 1338 reflections with I > 2σ(I) |
| Tmin = 0.612, Tmax = 0.872 | Rint = 0.028 |
| 11327 measured reflections | |
Refinement top
| R[F2 > 2σ(F2)] = 0.015 | 0 restraints |
| wR(F2) = 0.038 | H atoms treated by a mixture of independent and constrained refinement |
| S = 1.06 | Δρmax = 0.48 e Å−3 |
| 1355 reflections | Δρmin = −0.26 e Å−3 |
| 72 parameters | |
Special details top
Geometry. All e.s.d.'s (except the e.s.d. in the dihedral angle between two l.s. planes)
are estimated using the full covariance matrix. The cell e.s.d.'s are taken
into account individually in the estimation of e.s.d.'s in distances, angles
and torsion angles; correlations between e.s.d.'s in cell parameters are only
used when they are defined by crystal symmetry. An approximate (isotropic)
treatment of cell e.s.d.'s is used for estimating e.s.d.'s involving l.s.
planes. |
Refinement. Refinement of F2 against ALL reflections. The weighted R-factor
wR and goodness of fit S are based on F2, conventional
R-factors R are based on F, with F set to zero for
negative F2. The threshold expression of F2 >
σ(F2) is used only for calculating R-factors(gt) etc.
and is not relevant to the choice of reflections for refinement.
R-factors based on F2 are statistically about twice as large
as those based on F, and R- factors based on ALL data will be
even larger. |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2) top| | x | y | z | Uiso*/Ueq | |
| Zn1 | 0.6667 | 0.3333 | 0.26905 (3) | 0.00786 (6) | |
| N1 | 0.44778 (14) | 0.29824 (14) | 0.13945 (11) | 0.0123 (2) | |
| H1N | 0.423 (2) | 0.380 (2) | 0.1516 (16) | 0.014 (4)* | |
| H2N | 0.473 (2) | 0.302 (2) | 0.0548 (17) | 0.020 (4)* | |
| N2 | 0.45376 (13) | 0.12770 (14) | 0.38458 (10) | 0.01029 (17) | |
| H3N | 0.466 (2) | 0.150 (2) | 0.4679 (18) | 0.018 (4)* | |
| H4N | 0.455 (2) | 0.035 (2) | 0.3663 (15) | 0.009 (4)* | |
| C1 | 0.29803 (15) | 0.12899 (16) | 0.17684 (12) | 0.0135 (2) | |
| H1A | 0.3102 | 0.0343 | 0.1346 | 0.016* | |
| H1B | 0.1896 | 0.1209 | 0.1419 | 0.016* | |
| C2 | 0.28991 (15) | 0.11072 (16) | 0.33360 (12) | 0.0128 (2) | |
| H2A | 0.2715 | 0.2018 | 0.3754 | 0.015* | |
| H2B | 0.1914 | −0.0039 | 0.3602 | 0.015* | |
| S1 | 0.3333 | 0.6667 | 0.25564 (4) | 0.00718 (9) | |
| O1 | 0.31833 (13) | 0.50281 (11) | 0.30687 (9) | 0.01705 (18) | |
| O2 | 0.3333 | 0.6667 | 0.10375 (15) | 0.0242 (4) | |
Atomic displacement parameters (Å2) top| | U11 | U22 | U33 | U12 | U13 | U23 |
| Zn1 | 0.00806 (7) | 0.00806 (7) | 0.00747 (9) | 0.00403 (3) | 0.000 | 0.000 |
| N1 | 0.0128 (5) | 0.0145 (5) | 0.0102 (4) | 0.0072 (4) | −0.0002 (4) | 0.0006 (3) |
| N2 | 0.0110 (5) | 0.0098 (5) | 0.0100 (4) | 0.0051 (4) | 0.0004 (3) | −0.0002 (3) |
| C1 | 0.0099 (5) | 0.0142 (5) | 0.0138 (5) | 0.0041 (4) | −0.0038 (4) | −0.0024 (4) |
| C2 | 0.0093 (5) | 0.0129 (5) | 0.0143 (5) | 0.0040 (4) | 0.0011 (4) | 0.0001 (4) |
| S1 | 0.00719 (11) | 0.00719 (11) | 0.0071 (2) | 0.00360 (5) | 0.000 | 0.000 |
| O1 | 0.0199 (5) | 0.0117 (4) | 0.0216 (4) | 0.0094 (4) | −0.0003 (4) | 0.0028 (3) |
| O2 | 0.0331 (6) | 0.0331 (6) | 0.0063 (6) | 0.0165 (3) | 0.000 | 0.000 |
Geometric parameters (Å, º) top
| Zn1—N1 | 2.2035 (11) | N2—H4N | 0.847 (16) |
| Zn1—N1i | 2.2035 (11) | C1—C2 | 1.5246 (17) |
| Zn1—N1ii | 2.2035 (11) | C1—H1A | 0.9900 |
| Zn1—N2 | 2.1724 (10) | C1—H1B | 0.9900 |
| Zn1—N2ii | 2.1724 (10) | C2—H2A | 0.9900 |
| Zn1—N2i | 2.1724 (10) | C2—H2B | 0.9900 |
| N1—C1 | 1.4718 (15) | S1—O2 | 1.4711 (15) |
| N1—H1N | 0.869 (17) | S1—O1iii | 1.4809 (9) |
| N1—H2N | 0.846 (17) | S1—O1iv | 1.4810 (9) |
| N2—C2 | 1.4732 (16) | S1—O1 | 1.4810 (8) |
| N2—H3N | 0.824 (17) | | |
| | | |
| N2—Zn1—N2ii | 95.86 (3) | Zn1—N2—H3N | 110.4 (11) |
| N2—Zn1—N2i | 95.86 (3) | C2—N2—H4N | 108.7 (10) |
| N2ii—Zn1—N2i | 95.86 (3) | Zn1—N2—H4N | 106.5 (10) |
| N1—Zn1—N2 | 80.66 (4) | H3N—N2—H4N | 111.9 (17) |
| N1—Zn1—N2ii | 170.58 (4) | N1—C1—C2 | 108.93 (9) |
| N2i—Zn1—N1 | 93.21 (4) | N1—C1—H1A | 109.9 |
| N2—Zn1—N1i | 170.58 (4) | C2—C1—H1A | 109.9 |
| N2ii—Zn1—N1i | 93.21 (4) | N1—C1—H1B | 109.9 |
| N2i—Zn1—N1i | 80.66 (4) | C2—C1—H1B | 109.9 |
| N1—Zn1—N1i | 90.76 (4) | H1A—C1—H1B | 108.3 |
| N2—Zn1—N1ii | 93.21 (4) | N2—C2—C1 | 109.34 (9) |
| N2ii—Zn1—N1ii | 80.66 (4) | N2—C2—H2A | 109.8 |
| N2i—Zn1—N1ii | 170.58 (4) | C1—C2—H2A | 109.8 |
| N1—Zn1—N1ii | 90.76 (4) | N2—C2—H2B | 109.8 |
| N1i—Zn1—N1ii | 90.76 (4) | C1—C2—H2B | 109.8 |
| C1—N1—Zn1 | 106.76 (7) | H2A—C2—H2B | 108.3 |
| C1—N1—H1N | 110.0 (11) | O2—S1—O1iii | 109.57 (4) |
| Zn1—N1—H1N | 112.5 (10) | O2—S1—O1iv | 109.57 (4) |
| C1—N1—H2N | 111.2 (13) | O1iii—S1—O1iv | 109.37 (4) |
| Zn1—N1—H2N | 110.5 (11) | O2—S1—O1 | 109.57 (4) |
| H1N—N1—H2N | 105.9 (16) | O1iii—S1—O1 | 109.37 (4) |
| C2—N2—Zn1 | 108.35 (7) | O1iv—S1—O1 | 109.37 (4) |
| C2—N2—H3N | 110.9 (13) | | |
| | | |
| N2—Zn1—N1—C1 | −17.09 (8) | N1—Zn1—N2—C2 | −12.88 (7) |
| N2i—Zn1—N1—C1 | −112.51 (8) | N1ii—Zn1—N2—C2 | −103.12 (8) |
| N1i—Zn1—N1—C1 | 166.81 (7) | Zn1—N1—C1—C2 | 43.76 (11) |
| N1ii—Zn1—N1—C1 | 76.04 (9) | Zn1—N2—C2—C1 | 40.43 (11) |
| N2ii—Zn1—N2—C2 | 175.95 (8) | N1—C1—C2—N2 | −58.02 (13) |
| N2i—Zn1—N2—C2 | 79.42 (10) | | |
| Symmetry codes: (i) −x+y+1, −x+1, z; (ii) −y+1, x−y, z; (iii) −x+y, −x+1, z; (iv) −y+1, x−y+1, z. |
Hydrogen-bond geometry (Å, º) top
| D—H···A | D—H | H···A | D···A | D—H···A |
| N1—H1N···O1 | 0.869 (17) | 2.308 (17) | 3.0593 (14) | 144.8 (14) |
| N1—H2N···O2v | 0.846 (17) | 2.220 (17) | 2.9711 (16) | 148.0 (15) |
| N2—H3N···O1vi | 0.824 (17) | 2.205 (17) | 3.0257 (13) | 173.7 (19) |
| N2—H4N···O1ii | 0.847 (16) | 2.242 (17) | 3.0818 (16) | 171.5 (15) |
| Symmetry codes: (ii) −y+1, x−y, z; (v) −x+1, −y+1, −z; (vi) y, −x+y, −z+1. |
Crystal data top
| [Cu(C2H8N2)3]2+·SO42− | Z = 8 |
| Mr = 339.91 | F(000) = 1432 |
| Triclinic, A1 | Dx = 1.736 Mg m−3 |
| Hall symbol: -A 1 | Mo Kα radiation, λ = 0.71073 Å |
| a = 8.8749 (7) Å | Cell parameters from 5801 reflections |
| b = 17.6358 (11) Å | θ = 3.5–27.5° |
| c = 19.1559 (11) Å | µ = 1.86 mm−1 |
| α = 89.582 (3)° | T = 110 K |
| β = 90.296 (5)° | Needle, blue |
| γ = 119.845 (4)° | 0.33 × 0.15 × 0.06 mm |
| V = 2600.5 (3) Å3 | |
Data collection top
Nonius KappaCCD
diffractometer | 5825 independent reflections |
| Radiation source: rotating anode | 4478 reflections with I > 2σ(I) |
| Graphite monochromator | Rint = 0.033 |
| ϕ and ω scans | θmax = 27.8°, θmin = 1.7° |
Absorption correction: multi-scan
(TWINABS; Sheldrick, 2008a) | h = −11→11 |
| Tmin = 0.60, Tmax = 0.75 | k = −23→23 |
| 18964 measured reflections | l = −24→24 |
Refinement top
| Refinement on F2 | Primary atom site location: structure-invariant direct methods |
| Least-squares matrix: full | Secondary atom site location: difference Fourier map |
| R[F2 > 2σ(F2)] = 0.033 | Hydrogen site location: inferred from neighbouring sites |
| wR(F2) = 0.076 | H-atom parameters constrained |
| S = 1.02 | w = 1/[σ2(Fo2) + (0.0334P)2 + 3.8394P]
where P = (Fo2 + 2Fc2)/3 |
| 5825 reflections | (Δ/σ)max = 0.001 |
| 329 parameters | Δρmax = 0.77 e Å−3 |
| 0 restraints | Δρmin = −0.63 e Å−3 |
Crystal data top
| [Cu(C2H8N2)3]2+·SO42− | γ = 119.845 (4)° |
| Mr = 339.91 | V = 2600.5 (3) Å3 |
| Triclinic, A1 | Z = 8 |
| a = 8.8749 (7) Å | Mo Kα radiation |
| b = 17.6358 (11) Å | µ = 1.86 mm−1 |
| c = 19.1559 (11) Å | T = 110 K |
| α = 89.582 (3)° | 0.33 × 0.15 × 0.06 mm |
| β = 90.296 (5)° | |
Data collection top
Nonius KappaCCD
diffractometer | 5825 independent reflections |
Absorption correction: multi-scan
(TWINABS; Sheldrick, 2008a) | 4478 reflections with I > 2σ(I) |
| Tmin = 0.60, Tmax = 0.75 | Rint = 0.033 |
| 18964 measured reflections | |
Refinement top
| R[F2 > 2σ(F2)] = 0.033 | 0 restraints |
| wR(F2) = 0.076 | H-atom parameters constrained |
| S = 1.02 | Δρmax = 0.77 e Å−3 |
| 5825 reflections | Δρmin = −0.63 e Å−3 |
| 329 parameters | |
Special details top
Geometry. All e.s.d.'s (except the e.s.d. in the dihedral angle between two l.s. planes)
are estimated using the full covariance matrix. The cell e.s.d.'s are taken
into account individually in the estimation of e.s.d.'s in distances, angles
and torsion angles; correlations between e.s.d.'s in cell parameters are only
used when they are defined by crystal symmetry. An approximate (isotropic)
treatment of cell e.s.d.'s is used for estimating e.s.d.'s involving l.s.
planes. |
Refinement. Refinement of F2 against ALL reflections. The weighted R-factor
wR and goodness of fit S are based on F2, conventional
R-factors R are based on F, with F set to zero for
negative F2. The threshold expression of F2 >
σ(F2) is used only for calculating R-factors(gt) etc.
and is not relevant to the choice of reflections for refinement.
R-factors based on F2 are statistically about twice as large
as those based on F, and R- factors based on ALL data will be
even larger. |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2) top| | x | y | z | Uiso*/Ueq | |
| Cu1 | 0.33615 (6) | 0.33809 (4) | 0.12285 (3) | 0.00794 (10) | |
| N1 | 0.5694 (4) | 0.4426 (2) | 0.05170 (18) | 0.0123 (8) | |
| H1N | 0.5537 | 0.4261 | 0.0056 | 0.015* | |
| H2N | 0.5815 | 0.4973 | 0.0553 | 0.015* | |
| N2 | 0.5460 (4) | 0.3584 (2) | 0.18329 (17) | 0.0116 (7) | |
| H3N | 0.5234 | 0.3634 | 0.2293 | 0.014* | |
| H4N | 0.5623 | 0.3110 | 0.1796 | 0.014* | |
| N3 | 0.3161 (5) | 0.4319 (2) | 0.17775 (17) | 0.0117 (7) | |
| H5N | 0.3283 | 0.4254 | 0.2248 | 0.014* | |
| H6N | 0.4017 | 0.4867 | 0.1642 | 0.014* | |
| N4 | 0.1334 (4) | 0.3322 (2) | 0.06618 (18) | 0.0118 (8) | |
| H7N | 0.1564 | 0.3345 | 0.0192 | 0.014* | |
| H8N | 0.0331 | 0.2805 | 0.0755 | 0.014* | |
| N5 | 0.3296 (5) | 0.2359 (2) | 0.06962 (16) | 0.0119 (7) | |
| H9N | 0.3213 | 0.2436 | 0.0225 | 0.014* | |
| H10N | 0.4311 | 0.2351 | 0.0776 | 0.014* | |
| N6 | 0.1473 (5) | 0.2203 (2) | 0.19669 (18) | 0.0152 (8) | |
| H11N | 0.1801 | 0.2322 | 0.2427 | 0.018* | |
| H12N | 0.0341 | 0.2080 | 0.1920 | 0.018* | |
| C1 | 0.7180 (5) | 0.4399 (3) | 0.0821 (2) | 0.0139 (9) | |
| H1A | 0.8272 | 0.4921 | 0.0672 | 0.017* | |
| H1B | 0.7199 | 0.3873 | 0.0657 | 0.017* | |
| C2 | 0.7047 (5) | 0.4378 (3) | 0.1615 (2) | 0.0139 (9) | |
| H2A | 0.8075 | 0.4383 | 0.1823 | 0.017* | |
| H2B | 0.7018 | 0.4901 | 0.1779 | 0.017* | |
| C3 | 0.1422 (5) | 0.4212 (3) | 0.1631 (2) | 0.0124 (9) | |
| H3A | 0.1389 | 0.4743 | 0.1770 | 0.015* | |
| H3B | 0.0516 | 0.3707 | 0.1894 | 0.015* | |
| C4 | 0.1112 (6) | 0.4065 (3) | 0.0856 (2) | 0.0140 (9) | |
| H4A | −0.0078 | 0.3938 | 0.0738 | 0.017* | |
| H4B | 0.1948 | 0.4595 | 0.0596 | 0.017* | |
| C5 | 0.1809 (5) | 0.1519 (3) | 0.0919 (2) | 0.0137 (9) | |
| H5A | 0.1952 | 0.1031 | 0.0744 | 0.016* | |
| H5B | 0.0722 | 0.1457 | 0.0720 | 0.016* | |
| C6 | 0.1693 (6) | 0.1477 (3) | 0.1708 (2) | 0.0149 (9) | |
| H6A | 0.0694 | 0.0911 | 0.1856 | 0.018* | |
| H6B | 0.2764 | 0.1520 | 0.1907 | 0.018* | |
| Cu2 | 0.32986 (6) | 0.33160 (4) | 0.62178 (3) | 0.00803 (10) | |
| N7 | 0.1283 (5) | 0.3483 (2) | 0.55075 (18) | 0.0138 (8) | |
| H13N | 0.1648 | 0.3597 | 0.5051 | 0.017* | |
| H14N | 0.0199 | 0.2993 | 0.5525 | 0.017* | |
| N8 | 0.2854 (4) | 0.4119 (2) | 0.68437 (18) | 0.0143 (8) | |
| H15N | 0.2717 | 0.3922 | 0.7298 | 0.017* | |
| H16N | 0.3796 | 0.4675 | 0.6827 | 0.017* | |
| N9 | 0.1434 (4) | 0.2236 (2) | 0.67346 (18) | 0.0120 (8) | |
| H17N | 0.1593 | 0.2313 | 0.7209 | 0.014* | |
| H18N | 0.0347 | 0.2146 | 0.6625 | 0.014* | |
| N10 | 0.3447 (5) | 0.2414 (2) | 0.55943 (16) | 0.0120 (7) | |
| H19N | 0.3460 | 0.2561 | 0.5132 | 0.014* | |
| H20N | 0.4448 | 0.2399 | 0.5690 | 0.014* | |
| N11 | 0.5369 (5) | 0.4353 (2) | 0.57148 (19) | 0.0126 (8) | |
| H21N | 0.5232 | 0.4274 | 0.5240 | 0.015* | |
| H22N | 0.5383 | 0.4864 | 0.5821 | 0.015* | |
| N12 | 0.5659 (5) | 0.3477 (2) | 0.69349 (19) | 0.0168 (8) | |
| H23N | 0.5413 | 0.3472 | 0.7401 | 0.020* | |
| H24N | 0.5921 | 0.3043 | 0.6849 | 0.020* | |
| C7 | 0.1281 (6) | 0.4229 (3) | 0.5824 (2) | 0.0150 (9) | |
| H7A | 0.0239 | 0.4254 | 0.5671 | 0.018* | |
| H7B | 0.2324 | 0.4776 | 0.5674 | 0.018* | |
| C8 | 0.1278 (5) | 0.4140 (3) | 0.6616 (2) | 0.0144 (9) | |
| H8A | 0.1253 | 0.4640 | 0.6836 | 0.017* | |
| H8B | 0.0228 | 0.3595 | 0.6766 | 0.017* | |
| C9 | 0.1594 (5) | 0.1473 (2) | 0.6515 (2) | 0.0136 (9) | |
| H9A | 0.0512 | 0.0922 | 0.6623 | 0.016* | |
| H9B | 0.2570 | 0.1469 | 0.6764 | 0.016* | |
| C10 | 0.1922 (5) | 0.1558 (3) | 0.5738 (2) | 0.0128 (9) | |
| H10A | 0.2132 | 0.1087 | 0.5576 | 0.015* | |
| H10B | 0.0896 | 0.1506 | 0.5488 | 0.015* | |
| C11 | 0.7034 (5) | 0.4422 (3) | 0.5928 (2) | 0.0165 (9) | |
| H11A | 0.8011 | 0.4994 | 0.5779 | 0.020* | |
| H11B | 0.7168 | 0.3956 | 0.5701 | 0.020* | |
| C12 | 0.7069 (5) | 0.4330 (3) | 0.6717 (2) | 0.0157 (9) | |
| H12A | 0.8197 | 0.4393 | 0.6863 | 0.019* | |
| H12B | 0.6940 | 0.4797 | 0.6943 | 0.019* | |
| S1 | 0.34565 (13) | 0.33906 (8) | 0.37088 (6) | 0.00867 (17) | |
| O1 | 0.1843 (4) | 0.33810 (19) | 0.38979 (15) | 0.0205 (7) | |
| O2 | 0.4601 (4) | 0.3625 (2) | 0.43202 (15) | 0.0290 (8) | |
| O3 | 0.4363 (4) | 0.40648 (17) | 0.31547 (14) | 0.0203 (7) | |
| O4 | 0.3076 (4) | 0.25405 (18) | 0.34456 (16) | 0.0285 (8) | |
| S2 | 0.31922 (13) | 0.32563 (7) | 0.88148 (5) | 0.00915 (19) | |
| O5 | 0.4970 (4) | 0.39721 (19) | 0.89031 (19) | 0.0334 (9) | |
| O6 | 0.3007 (4) | 0.24660 (17) | 0.91696 (14) | 0.0178 (6) | |
| O7 | 0.1981 (4) | 0.3494 (2) | 0.91273 (15) | 0.0258 (7) | |
| O8 | 0.2764 (5) | 0.3063 (2) | 0.80775 (14) | 0.0385 (9) | |
Atomic displacement parameters (Å2) top| | U11 | U22 | U33 | U12 | U13 | U23 |
| Cu1 | 0.0082 (2) | 0.0108 (3) | 0.00574 (19) | 0.0055 (3) | −0.0018 (3) | −0.0012 (2) |
| N1 | 0.0148 (19) | 0.0149 (17) | 0.0102 (18) | 0.0096 (15) | −0.0016 (14) | −0.0004 (14) |
| N2 | 0.0100 (17) | 0.0156 (17) | 0.0085 (17) | 0.0059 (14) | 0.0027 (13) | 0.0012 (14) |
| N3 | 0.0133 (18) | 0.0092 (16) | 0.0103 (17) | 0.0040 (14) | −0.0016 (15) | 0.0013 (13) |
| N4 | 0.0097 (17) | 0.0158 (17) | 0.0090 (17) | 0.0055 (15) | 0.0006 (13) | −0.0011 (14) |
| N5 | 0.0142 (17) | 0.0141 (17) | 0.0078 (16) | 0.0072 (15) | 0.0006 (15) | 0.0008 (13) |
| N6 | 0.0162 (19) | 0.0158 (18) | 0.0096 (18) | 0.0051 (15) | 0.0015 (14) | 0.0006 (14) |
| C1 | 0.009 (2) | 0.0136 (19) | 0.015 (2) | 0.0024 (16) | 0.0005 (16) | 0.0003 (16) |
| C2 | 0.006 (2) | 0.016 (2) | 0.014 (2) | 0.0015 (17) | −0.0036 (16) | 0.0000 (16) |
| C3 | 0.014 (2) | 0.014 (2) | 0.010 (2) | 0.0081 (17) | 0.0019 (16) | 0.0000 (16) |
| C4 | 0.016 (2) | 0.018 (2) | 0.011 (2) | 0.0096 (18) | −0.0002 (17) | 0.0037 (16) |
| C5 | 0.015 (2) | 0.0128 (19) | 0.015 (2) | 0.0082 (18) | −0.0021 (17) | −0.0022 (17) |
| C6 | 0.020 (2) | 0.0105 (19) | 0.015 (2) | 0.0074 (18) | 0.0001 (17) | 0.0017 (16) |
| Cu2 | 0.0082 (2) | 0.0085 (3) | 0.00646 (19) | 0.0035 (3) | 0.0002 (3) | −0.0008 (2) |
| N7 | 0.0118 (18) | 0.0147 (17) | 0.0128 (18) | 0.0050 (15) | −0.0008 (14) | −0.0006 (14) |
| N8 | 0.0158 (19) | 0.0137 (16) | 0.0105 (17) | 0.0051 (15) | 0.0021 (14) | −0.0009 (13) |
| N9 | 0.0107 (17) | 0.0125 (16) | 0.0099 (18) | 0.0035 (14) | −0.0011 (14) | 0.0010 (13) |
| N10 | 0.0161 (18) | 0.0151 (16) | 0.0070 (16) | 0.0094 (15) | 0.0006 (14) | 0.0002 (13) |
| N11 | 0.0143 (18) | 0.0126 (16) | 0.0116 (18) | 0.0073 (15) | 0.0028 (14) | 0.0021 (14) |
| N12 | 0.015 (2) | 0.0238 (19) | 0.0125 (19) | 0.0099 (17) | −0.0001 (15) | 0.0001 (16) |
| C7 | 0.013 (2) | 0.015 (2) | 0.015 (2) | 0.0062 (17) | 0.0015 (17) | 0.0049 (16) |
| C8 | 0.010 (2) | 0.013 (2) | 0.020 (2) | 0.0063 (17) | 0.0022 (17) | −0.0025 (16) |
| C9 | 0.014 (2) | 0.0074 (18) | 0.015 (2) | 0.0027 (17) | 0.0001 (17) | −0.0003 (15) |
| C10 | 0.014 (2) | 0.0140 (19) | 0.010 (2) | 0.0068 (17) | −0.0053 (16) | −0.0079 (16) |
| C11 | 0.010 (2) | 0.014 (2) | 0.021 (2) | 0.0021 (17) | 0.0057 (17) | 0.0013 (17) |
| C12 | 0.009 (2) | 0.017 (2) | 0.020 (2) | 0.0049 (17) | −0.0053 (16) | −0.0059 (16) |
| S1 | 0.0097 (4) | 0.0101 (5) | 0.0066 (4) | 0.0052 (5) | −0.0008 (5) | −0.0009 (4) |
| O1 | 0.0128 (15) | 0.0256 (15) | 0.0240 (17) | 0.0101 (13) | 0.0036 (12) | 0.0013 (13) |
| O2 | 0.0236 (17) | 0.047 (2) | 0.0102 (15) | 0.0131 (15) | −0.0065 (13) | 0.0011 (14) |
| O3 | 0.0205 (16) | 0.0191 (14) | 0.0123 (15) | 0.0029 (12) | 0.0017 (12) | 0.0041 (11) |
| O4 | 0.0363 (19) | 0.0179 (15) | 0.0359 (19) | 0.0168 (15) | 0.0031 (16) | −0.0037 (13) |
| S2 | 0.0107 (4) | 0.0097 (5) | 0.0063 (5) | 0.0045 (5) | −0.0004 (5) | −0.0007 (4) |
| O5 | 0.0141 (16) | 0.0209 (16) | 0.056 (2) | 0.0020 (13) | −0.0035 (15) | 0.0107 (15) |
| O6 | 0.0210 (16) | 0.0147 (13) | 0.0180 (15) | 0.0091 (12) | 0.0008 (12) | 0.0011 (11) |
| O7 | 0.0294 (18) | 0.0345 (17) | 0.0244 (17) | 0.0241 (16) | 0.0099 (14) | 0.0070 (14) |
| O8 | 0.072 (3) | 0.0319 (18) | 0.0052 (14) | 0.0211 (18) | −0.0074 (15) | −0.0052 (12) |
Geometric parameters (Å, º) top
| Cu1—N1 | 2.395 (3) | Cu2—N11 | 2.073 (3) |
| Cu1—N2 | 2.063 (3) | Cu2—N12 | 2.396 (4) |
| Cu1—N3 | 2.048 (3) | N7—C7 | 1.452 (5) |
| Cu1—N4 | 2.055 (3) | N7—H13N | 0.9200 |
| Cu1—N5 | 2.052 (3) | N7—H14N | 0.9200 |
| Cu1—N6 | 2.375 (3) | N8—C8 | 1.480 (5) |
| N1—C1 | 1.461 (5) | N8—H15N | 0.9200 |
| N1—H1N | 0.9200 | N8—H16N | 0.9200 |
| N1—H2N | 0.9200 | N9—C9 | 1.484 (5) |
| N2—C2 | 1.468 (5) | N9—H17N | 0.9200 |
| N2—H3N | 0.9200 | N9—H18N | 0.9200 |
| N2—H4N | 0.9200 | N10—C10 | 1.466 (5) |
| N3—C3 | 1.484 (5) | N10—H19N | 0.9200 |
| N3—H5N | 0.9200 | N10—H20N | 0.9200 |
| N3—H6N | 0.9200 | N11—C11 | 1.477 (5) |
| N4—C4 | 1.468 (5) | N11—H21N | 0.9200 |
| N4—H7N | 0.9200 | N11—H22N | 0.9200 |
| N4—H8N | 0.9200 | N12—C12 | 1.458 (5) |
| N5—C5 | 1.473 (5) | N12—H23N | 0.9200 |
| N5—H9N | 0.9200 | N12—H24N | 0.9200 |
| N5—H10N | 0.9200 | C7—C8 | 1.525 (6) |
| N6—C6 | 1.478 (5) | C7—H7A | 0.9900 |
| N6—H11N | 0.9200 | C7—H7B | 0.9900 |
| N6—H12N | 0.9200 | C8—H8A | 0.9900 |
| C1—C2 | 1.523 (5) | C8—H8B | 0.9900 |
| C1—H1A | 0.9900 | C9—C10 | 1.509 (5) |
| C1—H1B | 0.9900 | C9—H9A | 0.9900 |
| C2—H2A | 0.9900 | C9—H9B | 0.9900 |
| C2—H2B | 0.9900 | C10—H10A | 0.9900 |
| C3—C4 | 1.509 (6) | C10—H10B | 0.9900 |
| C3—H3A | 0.9900 | C11—C12 | 1.520 (6) |
| C3—H3B | 0.9900 | C11—H11A | 0.9900 |
| C4—H4A | 0.9900 | C11—H11B | 0.9900 |
| C4—H4B | 0.9900 | C12—H12A | 0.9900 |
| C5—C6 | 1.513 (5) | C12—H12B | 0.9900 |
| C5—H5A | 0.9900 | S1—O4 | 1.456 (3) |
| C5—H5B | 0.9900 | S1—O2 | 1.466 (3) |
| C6—H6A | 0.9900 | S1—O1 | 1.470 (3) |
| C6—H6B | 0.9900 | S1—O3 | 1.492 (3) |
| Cu2—N7 | 2.373 (3) | S2—O8 | 1.459 (3) |
| Cu2—N8 | 2.045 (3) | S2—O5 | 1.459 (3) |
| Cu2—N9 | 2.049 (3) | S2—O7 | 1.465 (3) |
| Cu2—N10 | 2.052 (3) | S2—O6 | 1.480 (3) |
| | | |
| N1—Cu1—N2 | 79.82 (12) | N8—Cu2—N10 | 173.49 (15) |
| N1—Cu1—N3 | 93.62 (13) | N8—Cu2—N11 | 93.18 (14) |
| N1—Cu1—N4 | 98.10 (12) | N8—Cu2—N12 | 92.24 (13) |
| N1—Cu1—N5 | 91.48 (13) | N9—Cu2—N10 | 83.99 (14) |
| N1—Cu1—N6 | 166.73 (12) | N9—Cu2—N11 | 173.57 (13) |
| N2—Cu1—N3 | 90.21 (14) | N9—Cu2—N12 | 95.18 (13) |
| N2—Cu1—N4 | 173.29 (14) | N10—Cu2—N11 | 92.35 (14) |
| N2—Cu1—N5 | 93.55 (14) | N10—Cu2—N12 | 92.15 (14) |
| N2—Cu1—N6 | 90.26 (13) | N11—Cu2—N12 | 79.64 (12) |
| N3—Cu1—N4 | 83.53 (14) | C7—N7—Cu2 | 102.2 (2) |
| N3—Cu1—N5 | 174.13 (12) | C7—N7—H13N | 111.3 |
| N3—Cu1—N6 | 95.21 (13) | Cu2—N7—H13N | 111.3 |
| N4—Cu1—N5 | 92.88 (14) | C7—N7—H14N | 111.3 |
| N4—Cu1—N6 | 92.71 (13) | Cu2—N7—H14N | 111.3 |
| N5—Cu1—N6 | 80.27 (13) | H13N—N7—H14N | 109.2 |
| C1—N1—Cu1 | 101.5 (2) | C8—N8—Cu2 | 110.9 (3) |
| C1—N1—H1N | 111.5 | C8—N8—H15N | 109.5 |
| Cu1—N1—H1N | 111.5 | Cu2—N8—H15N | 109.5 |
| C1—N1—H2N | 111.5 | C8—N8—H16N | 109.5 |
| Cu1—N1—H2N | 111.5 | Cu2—N8—H16N | 109.5 |
| H1N—N1—H2N | 109.3 | H15N—N8—H16N | 108.1 |
| C2—N2—Cu1 | 111.3 (2) | C9—N9—Cu2 | 107.8 (2) |
| C2—N2—H3N | 109.4 | C9—N9—H17N | 110.1 |
| Cu1—N2—H3N | 109.4 | Cu2—N9—H17N | 110.1 |
| C2—N2—H4N | 109.4 | C9—N9—H18N | 110.1 |
| Cu1—N2—H4N | 109.4 | Cu2—N9—H18N | 110.1 |
| H3N—N2—H4N | 108.0 | H17N—N9—H18N | 108.5 |
| C3—N3—Cu1 | 107.7 (2) | C10—N10—Cu2 | 107.9 (2) |
| C3—N3—H5N | 110.2 | C10—N10—H19N | 110.1 |
| Cu1—N3—H5N | 110.2 | Cu2—N10—H19N | 110.1 |
| C3—N3—H6N | 110.2 | C10—N10—H20N | 110.1 |
| Cu1—N3—H6N | 110.2 | Cu2—N10—H20N | 110.1 |
| H5N—N3—H6N | 108.5 | H19N—N10—H20N | 108.4 |
| C4—N4—Cu1 | 108.8 (3) | C11—N11—Cu2 | 111.0 (2) |
| C4—N4—H7N | 109.9 | C11—N11—H21N | 109.4 |
| Cu1—N4—H7N | 109.9 | Cu2—N11—H21N | 109.4 |
| C4—N4—H8N | 109.9 | C11—N11—H22N | 109.4 |
| Cu1—N4—H8N | 109.9 | Cu2—N11—H22N | 109.4 |
| H7N—N4—H8N | 108.3 | H21N—N11—H22N | 108.0 |
| C5—N5—Cu1 | 111.0 (2) | C12—N12—Cu2 | 102.2 (2) |
| C5—N5—H9N | 109.4 | C12—N12—H23N | 111.3 |
| Cu1—N5—H9N | 109.4 | Cu2—N12—H23N | 111.3 |
| C5—N5—H10N | 109.4 | C12—N12—H24N | 111.3 |
| Cu1—N5—H10N | 109.4 | Cu2—N12—H24N | 111.3 |
| H9N—N5—H10N | 108.0 | H23N—N12—H24N | 109.2 |
| C6—N6—Cu1 | 102.4 (2) | N7—C7—C8 | 109.2 (3) |
| C6—N6—H11N | 111.3 | N7—C7—H7A | 109.8 |
| Cu1—N6—H11N | 111.3 | C8—C7—H7A | 109.8 |
| C6—N6—H12N | 111.3 | N7—C7—H7B | 109.8 |
| Cu1—N6—H12N | 111.3 | C8—C7—H7B | 109.8 |
| H11N—N6—H12N | 109.2 | H7A—C7—H7B | 108.3 |
| N1—C1—C2 | 109.5 (3) | N8—C8—C7 | 109.7 (3) |
| N1—C1—H1A | 109.8 | N8—C8—H8A | 109.7 |
| C2—C1—H1A | 109.8 | C7—C8—H8A | 109.7 |
| N1—C1—H1B | 109.8 | N8—C8—H8B | 109.7 |
| C2—C1—H1B | 109.8 | C7—C8—H8B | 109.7 |
| H1A—C1—H1B | 108.2 | H8A—C8—H8B | 108.2 |
| N2—C2—C1 | 109.4 (3) | N9—C9—C10 | 107.3 (3) |
| N2—C2—H2A | 109.8 | N9—C9—H9A | 110.3 |
| C1—C2—H2A | 109.8 | C10—C9—H9A | 110.3 |
| N2—C2—H2B | 109.8 | N9—C9—H9B | 110.3 |
| C1—C2—H2B | 109.8 | C10—C9—H9B | 110.3 |
| H2A—C2—H2B | 108.2 | H9A—C9—H9B | 108.5 |
| N3—C3—C4 | 107.0 (3) | N10—C10—C9 | 108.6 (3) |
| N3—C3—H3A | 110.3 | N10—C10—H10A | 110.0 |
| C4—C3—H3A | 110.3 | C9—C10—H10A | 110.0 |
| N3—C3—H3B | 110.3 | N10—C10—H10B | 110.0 |
| C4—C3—H3B | 110.3 | C9—C10—H10B | 110.0 |
| H3A—C3—H3B | 108.6 | H10A—C10—H10B | 108.4 |
| N4—C4—C3 | 108.4 (3) | N11—C11—C12 | 109.5 (4) |
| N4—C4—H4A | 110.0 | N11—C11—H11A | 109.8 |
| C3—C4—H4A | 110.0 | C12—C11—H11A | 109.8 |
| N4—C4—H4B | 110.0 | N11—C11—H11B | 109.8 |
| C3—C4—H4B | 110.0 | C12—C11—H11B | 109.8 |
| H4A—C4—H4B | 108.4 | H11A—C11—H11B | 108.2 |
| N5—C5—C6 | 109.9 (3) | N12—C12—C11 | 109.8 (3) |
| N5—C5—H5A | 109.7 | N12—C12—H12A | 109.7 |
| C6—C5—H5A | 109.7 | C11—C12—H12A | 109.7 |
| N5—C5—H5B | 109.7 | N12—C12—H12B | 109.7 |
| C6—C5—H5B | 109.7 | C11—C12—H12B | 109.7 |
| H5A—C5—H5B | 108.2 | H12A—C12—H12B | 108.2 |
| N6—C6—C5 | 109.6 (3) | O4—S1—O2 | 110.2 (2) |
| N6—C6—H6A | 109.8 | O4—S1—O1 | 110.57 (18) |
| C5—C6—H6A | 109.8 | O2—S1—O1 | 109.66 (19) |
| N6—C6—H6B | 109.8 | O4—S1—O3 | 109.11 (18) |
| C5—C6—H6B | 109.8 | O2—S1—O3 | 108.47 (17) |
| H6A—C6—H6B | 108.2 | O1—S1—O3 | 108.83 (17) |
| N7—Cu2—N8 | 80.43 (13) | O8—S2—O5 | 111.0 (2) |
| N7—Cu2—N9 | 94.53 (12) | O8—S2—O7 | 109.0 (2) |
| N7—Cu2—N10 | 96.02 (13) | O5—S2—O7 | 109.6 (2) |
| N7—Cu2—N11 | 91.10 (13) | O8—S2—O6 | 108.94 (18) |
| N7—Cu2—N12 | 167.91 (13) | O5—S2—O6 | 109.11 (18) |
| N8—Cu2—N9 | 90.82 (14) | O7—S2—O6 | 109.14 (17) |
| | | |
| N3—Cu1—N1—C1 | 104.9 (2) | N8—Cu2—N7—C7 | 15.2 (2) |
| N5—Cu1—N1—C1 | −78.0 (2) | N9—Cu2—N7—C7 | 105.3 (2) |
| N4—Cu1—N1—C1 | −171.2 (2) | N10—Cu2—N7—C7 | −170.3 (2) |
| N2—Cu1—N1—C1 | 15.3 (2) | N11—Cu2—N7—C7 | −77.8 (2) |
| N6—Cu1—N1—C1 | −26.8 (6) | N12—Cu2—N7—C7 | −38.0 (7) |
| N3—Cu1—N2—C2 | −77.8 (3) | N9—Cu2—N8—C8 | −79.0 (3) |
| N5—Cu1—N2—C2 | 106.7 (3) | N11—Cu2—N8—C8 | 106.0 (3) |
| N6—Cu1—N2—C2 | −173.1 (3) | N7—Cu2—N8—C8 | 15.5 (2) |
| N1—Cu1—N2—C2 | 15.8 (3) | N12—Cu2—N8—C8 | −174.2 (3) |
| N4—Cu1—N3—C3 | 18.4 (2) | N8—Cu2—N9—C9 | −168.5 (3) |
| N2—Cu1—N3—C3 | −164.0 (2) | N10—Cu2—N9—C9 | 15.5 (3) |
| N6—Cu1—N3—C3 | −73.8 (2) | N7—Cu2—N9—C9 | 111.0 (3) |
| N1—Cu1—N3—C3 | 116.1 (2) | N12—Cu2—N9—C9 | −76.2 (3) |
| N3—Cu1—N4—C4 | 11.1 (2) | N9—Cu2—N10—C10 | 14.0 (3) |
| N5—Cu1—N4—C4 | −173.6 (2) | N11—Cu2—N10—C10 | −171.3 (3) |
| N6—Cu1—N4—C4 | 106.0 (2) | N7—Cu2—N10—C10 | −80.0 (3) |
| N1—Cu1—N4—C4 | −81.7 (2) | N12—Cu2—N10—C10 | 109.0 (3) |
| N4—Cu1—N5—C5 | −75.0 (3) | N8—Cu2—N11—C11 | 108.3 (3) |
| N2—Cu1—N5—C5 | 107.0 (3) | N10—Cu2—N11—C11 | −75.2 (3) |
| N6—Cu1—N5—C5 | 17.3 (3) | N7—Cu2—N11—C11 | −171.3 (3) |
| N1—Cu1—N5—C5 | −173.1 (3) | N12—Cu2—N11—C11 | 16.6 (3) |
| N3—Cu1—N6—C6 | −171.0 (3) | N8—Cu2—N12—C12 | −78.7 (3) |
| N5—Cu1—N6—C6 | 12.8 (3) | N9—Cu2—N12—C12 | −169.7 (3) |
| N4—Cu1—N6—C6 | 105.2 (3) | N10—Cu2—N12—C12 | 106.2 (3) |
| N2—Cu1—N6—C6 | −80.8 (3) | N11—Cu2—N12—C12 | 14.2 (3) |
| N1—Cu1—N6—C6 | −39.5 (6) | N7—Cu2—N12—C12 | −26.4 (7) |
| Cu1—N1—C1—C2 | −42.6 (3) | Cu2—N7—C7—C8 | −42.0 (3) |
| Cu1—N2—C2—C1 | −45.1 (4) | Cu2—N8—C8—C7 | −44.3 (4) |
| N1—C1—C2—N2 | 61.6 (4) | N7—C7—C8—N8 | 60.5 (4) |
| Cu1—N3—C3—C4 | −43.7 (3) | Cu2—N9—C9—C10 | −41.3 (4) |
| Cu1—N4—C4—C3 | −38.2 (4) | Cu2—N10—C10—C9 | −40.7 (4) |
| N3—C3—C4—N4 | 54.7 (4) | N9—C9—C10—N10 | 55.1 (4) |
| Cu1—N5—C5—C6 | −46.0 (4) | Cu2—N11—C11—C12 | −45.5 (4) |
| Cu1—N6—C6—C5 | −39.8 (4) | Cu2—N12—C12—C11 | −41.6 (4) |
| N5—C5—C6—N6 | 59.5 (4) | N11—C11—C12—N12 | 61.0 (4) |
Hydrogen-bond geometry (Å, º) top
| D—H···A | D—H | H···A | D···A | D—H···A |
| N1—H1N···O5i | 0.92 | 2.27 | 3.180 (5) | 172 |
| N1—H2N···O5ii | 0.92 | 2.52 | 3.358 (5) | 151 |
| N1—H2N···O7ii | 0.92 | 2.50 | 3.267 (5) | 141 |
| N2—H3N···O3 | 0.92 | 2.12 | 2.993 (4) | 157 |
| N2—H4N···O4iii | 0.92 | 2.05 | 2.914 (4) | 156 |
| N3—H5N···O3 | 0.92 | 2.08 | 2.954 (4) | 158 |
| N3—H6N···O5ii | 0.92 | 2.06 | 2.920 (4) | 155 |
| N4—H7N···O7i | 0.92 | 2.07 | 2.982 (4) | 174 |
| N4—H8N···O1iv | 0.92 | 2.13 | 3.040 (4) | 172 |
| N5—H9N···O6i | 0.92 | 2.03 | 2.947 (4) | 173 |
| N5—H10N···O2iii | 0.92 | 2.37 | 3.119 (5) | 139 |
| N6—H11N···O4 | 0.92 | 2.19 | 3.089 (5) | 166 |
| N6—H12N···O1iv | 0.92 | 2.30 | 3.070 (5) | 141 |
| N7—H13N···O1 | 0.92 | 2.27 | 3.146 (5) | 160 |
| N7—H14N···O7v | 0.92 | 2.45 | 3.312 (5) | 156 |
| N8—H15N···O8 | 0.92 | 2.14 | 2.975 (5) | 151 |
| N8—H16N···O3ii | 0.92 | 2.00 | 2.914 (4) | 172 |
| N9—H17N···O8 | 0.92 | 2.06 | 2.907 (4) | 152 |
| N9—H18N···O7v | 0.92 | 2.30 | 3.109 (5) | 146 |
| N10—H19N···O2 | 0.92 | 2.25 | 3.056 (4) | 147 |
| N10—H20N···O6vi | 0.92 | 2.17 | 3.079 (5) | 172 |
| N11—H21N···O2 | 0.92 | 2.03 | 2.900 (5) | 158 |
| N11—H22N···O2ii | 0.92 | 2.67 | 3.552 (5) | 161 |
| N11—H22N···O3ii | 0.92 | 2.66 | 3.458 (5) | 145 |
| N12—H23N···O8 | 0.92 | 2.46 | 3.177 (5) | 135 |
| N12—H24N···O6vi | 0.92 | 2.53 | 3.268 (5) | 137 |
| Symmetry codes: (i) x, y, z−1; (ii) −x+1, −y+1, −z+1; (iii) −x+1, −y+1/2, −z+1/2; (iv) −x, −y+1/2, −z+1/2; (v) −x, −y+1/2, −z+3/2; (vi) −x+1, −y+1/2, −z+3/2. |
Experimental details
| | (I) | (II) |
| Crystal data |
| Chemical formula | [Zn(C2H8N2)3]2+·SO42− | [Cu(C2H8N2)3]2+·SO42− |
| Mr | 341.74 | 339.91 |
| Crystal system, space group | Trigonal, P3 | Triclinic, A1 |
| Temperature (K) | 110 | 110 |
| a, b, c (Å) | 8.89410 (11), 8.89410 (11), 9.68482 (13) | 8.8749 (7), 17.6358 (11), 19.1559 (11) |
| α, β, γ (°) | 90, 90, 120 | 89.582 (3), 90.296 (5), 119.845 (4) |
| V (Å3) | 663.48 (2) | 2600.5 (3) |
| Z | 2 | 8 |
| Radiation type | Mo Kα | Mo Kα |
| µ (mm−1) | 2.03 | 1.86 |
| Crystal size (mm) | 0.30 × 0.09 × 0.09 | 0.33 × 0.15 × 0.06 |
| |
| Data collection |
| Diffractometer | Nonius KappaCCD
diffractometer | Nonius KappaCCD
diffractometer |
| Absorption correction | Analytical
(SADABS; Sheldrick, 2008a) | Multi-scan
(TWINABS; Sheldrick, 2008a) |
| Tmin, Tmax | 0.612, 0.872 | 0.60, 0.75 |
No. of measured, independent and
observed [I > 2σ(I)] reflections | 11327, 1355, 1338 | 18964, 5825, 4478 |
| Rint | 0.028 | 0.033 |
| (sin θ/λ)max (Å−1) | 0.714 | 0.656 |
| |
| Refinement |
| R[F2 > 2σ(F2)], wR(F2), S | 0.015, 0.038, 1.06 | 0.033, 0.076, 1.02 |
| No. of reflections | 1355 | 5825 |
| No. of parameters | 72 | 329 |
| H-atom treatment | H atoms treated by a mixture of independent and constrained refinement | H-atom parameters constrained |
| Δρmax, Δρmin (e Å−3) | 0.48, −0.26 | 0.77, −0.63 |
Selected geometric parameters (Å, º) for (I) top| Zn1—N1 | 2.2035 (11) | N2—C2 | 1.4732 (16) |
| Zn1—N2 | 2.1724 (10) | C1—C2 | 1.5246 (17) |
| N1—C1 | 1.4718 (15) | | |
| | | |
| N1—Zn1—N2 | 80.66 (4) | C1—N1—Zn1 | 106.76 (7) |
| N1—Zn1—N2i | 170.58 (4) | C2—N2—Zn1 | 108.35 (7) |
| Symmetry code: (i) −y+1, x−y, z. |
Hydrogen-bond geometry (Å, º) for (I) top
| D—H···A | D—H | H···A | D···A | D—H···A |
| N1—H1N···O1 | 0.869 (17) | 2.308 (17) | 3.0593 (14) | 144.8 (14) |
| N1—H2N···O2ii | 0.846 (17) | 2.220 (17) | 2.9711 (16) | 148.0 (15) |
| N2—H3N···O1iii | 0.824 (17) | 2.205 (17) | 3.0257 (13) | 173.7 (19) |
| N2—H4N···O1i | 0.847 (16) | 2.242 (17) | 3.0818 (16) | 171.5 (15) |
| Symmetry codes: (i) −y+1, x−y, z; (ii) −x+1, −y+1, −z; (iii) y, −x+y, −z+1. |
Selected geometric parameters (Å, º) for (II) top| Cu1—N1 | 2.395 (3) | Cu2—N7 | 2.373 (3) |
| Cu1—N2 | 2.063 (3) | Cu2—N8 | 2.045 (3) |
| Cu1—N3 | 2.048 (3) | Cu2—N9 | 2.049 (3) |
| Cu1—N4 | 2.055 (3) | Cu2—N10 | 2.052 (3) |
| Cu1—N5 | 2.052 (3) | Cu2—N11 | 2.073 (3) |
| Cu1—N6 | 2.375 (3) | Cu2—N12 | 2.396 (4) |
| | | |
| N1—Cu1—N6 | 166.73 (12) | N7—Cu2—N12 | 167.91 (13) |
| N2—Cu1—N4 | 173.29 (14) | N8—Cu2—N10 | 173.49 (15) |
| N3—Cu1—N5 | 174.13 (12) | N9—Cu2—N11 | 173.57 (13) |
Hydrogen-bond geometry (Å, º) for (II) top
| D—H···A | D—H | H···A | D···A | D—H···A |
| N1—H1N···O5i | 0.92 | 2.27 | 3.180 (5) | 172 |
| N1—H2N···O5ii | 0.92 | 2.52 | 3.358 (5) | 151 |
| N1—H2N···O7ii | 0.92 | 2.50 | 3.267 (5) | 141 |
| N2—H3N···O3 | 0.92 | 2.12 | 2.993 (4) | 157 |
| N2—H4N···O4iii | 0.92 | 2.05 | 2.914 (4) | 156 |
| N3—H5N···O3 | 0.92 | 2.08 | 2.954 (4) | 158 |
| N3—H6N···O5ii | 0.92 | 2.06 | 2.920 (4) | 155 |
| N4—H7N···O7i | 0.92 | 2.07 | 2.982 (4) | 174 |
| N4—H8N···O1iv | 0.92 | 2.13 | 3.040 (4) | 172 |
| N5—H9N···O6i | 0.92 | 2.03 | 2.947 (4) | 173 |
| N5—H10N···O2iii | 0.92 | 2.37 | 3.119 (5) | 139 |
| N6—H11N···O4 | 0.92 | 2.19 | 3.089 (5) | 166 |
| N6—H12N···O1iv | 0.92 | 2.30 | 3.070 (5) | 141 |
| N7—H13N···O1 | 0.92 | 2.27 | 3.146 (5) | 160 |
| N7—H14N···O7v | 0.92 | 2.45 | 3.312 (5) | 156 |
| N8—H15N···O8 | 0.92 | 2.14 | 2.975 (5) | 151 |
| N8—H16N···O3ii | 0.92 | 2.00 | 2.914 (4) | 172 |
| N9—H17N···O8 | 0.92 | 2.06 | 2.907 (4) | 152 |
| N9—H18N···O7v | 0.92 | 2.30 | 3.109 (5) | 146 |
| N10—H19N···O2 | 0.92 | 2.25 | 3.056 (4) | 147 |
| N10—H20N···O6vi | 0.92 | 2.17 | 3.079 (5) | 172 |
| N11—H21N···O2 | 0.92 | 2.03 | 2.900 (5) | 158 |
| N11—H22N···O2ii | 0.92 | 2.67 | 3.552 (5) | 161 |
| N11—H22N···O3ii | 0.92 | 2.66 | 3.458 (5) | 145 |
| N12—H23N···O8 | 0.92 | 2.46 | 3.177 (5) | 135 |
| N12—H24N···O6vi | 0.92 | 2.53 | 3.268 (5) | 137 |
| Symmetry codes: (i) x, y, z−1; (ii) −x+1, −y+1, −z+1; (iii) −x+1, −y+1/2, −z+1/2; (iv) −x, −y+1/2, −z+1/2; (v) −x, −y+1/2, −z+3/2; (vi) −x+1, −y+1/2, −z+3/2. |
Equivalent twin matrices for inclusion in the least-squares refinement of (I).
The matrices were obtained by a coset decomposition (Flack, 1987) of
6/mmm with
respect to -3 using the Bilbao
Crystallographic Server (Aroyo et al., 2006). The twin matrix
with respect to
(hkl) is the transpose of the (xyz) matrix of the coordinates. top| twin matrix (hkl) | determinant | axis |
| 0 -1 0 / -1 0 0 / 0 0 -1 | +1 | 2 about (1 -1 0) |
| -1 0 0 / 1 1 0 / 0 0 -1 | +1 | 2 about (0 1 0) |
| 1 1 0 / 0 -1 0 / 0 0 -1 | +1 | 2 about (1 0 0) |
| 0 1 0 / 1 0 0 / 0 0 1 | -1 | m perpendicular to (1 -1 0) |
| 1 0 0 / -1 -1 0 / 0 0 1 | -1 | m perpendicular to (0 1 0) |
| -1 -1 0 / 0 1 0 / 0 0 1 | -1 | m perpendicular to (1 0 0) |
Relationship between the trigonal room temperature cell of (II) (Cullen
and Lingafelter, 1970) and the different possibilities to index the
triclinic structure at 110 K. top | room temperature | approximate supercell at 110 K | true cell at 110 K |
| non-standard setting | Bravais = P | Bravais = A | Bravais = A |
| a = 8.966 (1), b = 8.966 (1), c = 9.597 (1) Å | a = 17.7495 (6), b = 17.6371 (9), c = 19.1581 (10) Å | a = 8.8749 (7), b = 17.6358 (11), c = 19.1559 (11) Å |
| α = 90, β = 90, γ = 120 ° | α = 89.585 (4), β = 90.239 (3), γ = 119.687 (2) ° | α = 89.582 (3), β = 90.296 (5), γ = 119.845 (4) ° |
| V = 668.13 (13) Å3 | V = 5200.7 (3) Å3 | V = 2600.5 (3) Å3 |
| standard setting | Bravais = P | Bravais = C | Bravais = P |
| a = 8.966 (1), b = 8.966 (1), c = 9.597 (1) Å | a = 17.6371 (9), b = 30.7843 (12), c = 12.9731 (6) Å | a = 8.8749 (7), b = 12.9715 (6), c = 13.0660 (9) Å |
| α = 90, β = 90, γ = 120 ° | α = 89.976 (2), β = 132.408 (2), γ = 90.076 (2) ° | α = 71.959 (5), β = 70.148 (5), γ = 70.459 (5) ° |
| V = 668.13 (13) Å3 | V = 5200.7 (3) Å3 | V = 1300.25 (16) Å3 |
| reduced setting | Bravais = P | Bravais = P | Bravais = P |
| a = 8.966 (1), b = 8.966 (1), c = 9.597 (1) Å | a = 12.9731 (6), b = 13.0671 (8), c = 17.7292 (6) Å | a = 8.8749 (7), b = 12.9715 (6), c = 13.0660 (9) Å |
| α = 90, β = 90, γ = 120 ° | α = 109.702 (4), β = 109.578 (2), γ = 94.735 (4) ° | α = 71.959 (5), β = 70.148 (5), γ = 70.459 (5) ° |
| V = 668.13 (13) Å3 | V = 2600.4 (2) Å3 | V = 1300.25 (16) Å3 |

 journal menu
journal menu








![[Figure 1]](http://journals.iucr.org/c/issues/2010/11/00/gg3243/gg3243fig1thm.gif)
![[Figure 2]](http://journals.iucr.org/c/issues/2010/11/00/gg3243/gg3243fig2thm.gif)
![[Figure 3]](http://journals.iucr.org/c/issues/2010/11/00/gg3243/gg3243fig3thm.gif)
![[Figure 4]](http://journals.iucr.org/c/issues/2010/11/00/gg3243/gg3243fig4thm.gif)
![[Figure 5]](http://journals.iucr.org/c/issues/2010/11/00/gg3243/gg3243fig5thm.gif)




We report here the twinned crystal structures of [Zn(C2H8N2)3]2+.SO42-, (I), and [Cu(C2H8N2)3]2+.SO42-, (II), both measured at 110 (2) K. At room temperature the corresponding complexes of vanadium (Daniels et al., 1995), cobalt (Yotnoi et al., 2010), nickel (Mazhar-ul-Haque et al., 1970), copper (Cullen & Lingafelter, 1970) and zinc (Neverov et al., 1990) are isostructural in the trigonal space group P31c and the metal complex is a racemic mixture of the δδδ and λλλ isomers. There has also been a report of an isostructural manganese complex (Lu, 2009), but because of the positive residual electron density on the site of the metal and the unusually short metal—nitrogen distance we regard this structure with due care. In P31c the mentioned [title?] compounds have both the cationic metal complex and the sulfate anion on sites of 32 (D3) symmetry, respectively. The tetrahedral sulfate cannot fulfil this symmetry and must therefore be disordered. Different disorder models have been used in the literature. The octahedral metal complexes can, in principle, have 32 (D3) symmetry, but in the case of Cu2+ with a d9 electron configuration one would expect different Cu—N distances because of the Jahn–Teller theorem (Procter et al., 1968; Bertini et al., 1977, 1979). Cullen & Lingafelter (1970) describe the presence of dynamic Jahn–Teller effects in the room-temperature structure of the Cu complex with some evidence in the displacement parameters of the N atoms. It is not uncommon to see average structures of Jahn–Teller distorted complexes in X-ray crystal structure determinations because of the long time scale of the experiment (Persson et al., 2002).
At 110 (2) K the structure refinement of (I) based on the literature coordinates in space group P31c failed. The R1 value remains above 25% and anisotropic refinement leads to non-positive definite displacement parameters. In the list of the most disagreeable reflections the values of Fo2 are significantly larger than Fc2, which can be considered as a warning sign for twinning (Herbst-Irmer & Sheldrick, 1998). Because splitting of reflections was not detected in the diffraction images, only merohedral twinning is possible. A test with the TWINROTMAT routine of the PLATON software (Spek, 2009) did not find suitable twin operations, here. Therefore, hexagonal twinning of the P31c symmetric structure can be excluded. A closer inspection of the reflections showed that the extinction conditions related to the c-glide plane of P31c are violated. This prompted us to lower the symmetry to space group P3, which is a maximal isomorphic subgroup. The Rint value improves from 0.101 to 0.028 and the R1 value improves from 0.265 to 0.183 (isotropic refinement). In the low-trigonal P3 the twofold rotations perpendicular to the c axis are potential twin operations. A new test with TWINROTMAT now indeed finds the correct twin relation as a twofold rotation about hkl = (100). Its symmetry-equivalent operations can be obtained by a coset decomposition (Flack, 1987) of point group 6/mmm with respect to 3 and they are of course also suitable (Table 5). If the correct twin operation is included in the refinement (Herbst-Irmer & Sheldrick, 1998) R1 improves to 0.015 and the anisotropic refinement is stable. The twin fraction refined to 0.3745 (9).
The twinned low-temperature structure of the zinc complex, (I), at 110 (2) K corresponds to the low- temperature structure of the nickel compound in space group P3 (Jameson et al., 1982), where twinning was detected as well. The phase transition of the nickel complex occurs at 180 (1) K (Jameson et al., 1982), while in the zinc complex we still observe P31c symmetry at 140 (2) K during cooling. The phase transition in (I) is reversible and P31c symmetry is regained at 140 (2) K after warming up. In the low-temperature P3 structure both the cationic complex and the sulfate anion are located on sites with 3 (C3) symmetry, which can be fulfilled by the octahedral cation and the tetrahedral anion without disorder (Fig. 1). Because of this symmetry the zinc complex, (I), has two independent N and C atoms, respectively, and the r.m.s. deviation from 32 (D3) symmetry is 0.0619 Å as calculated with MOLSYM (Pilati & Forni, 1998). Similar to the room-temperature structure the N—Zn—N angle in the same ethylenediamine ligand (`bite angle') is smaller than 90° and the trans angles are smaller than 180°. The Zn1—N2 distance is 0.0311 (15) Å shorter than the Zn1—N1 distance and the C2—N2—Zn1 angle is 1.59 (10)° larger than the corresponding C1—N1—Zn1 angle (Table 1). As Zn2+ has a d10 electron configuration, electronic reasons can be excluded for this symmetry breaking. It might be a consequence of different intermolecular hydrogen-bonding geometries: the hydrogen bonds involving N2 are more linear than those involving N1 (Table 2). While the five-membered chelate ring in the room-temperature phase is in an exact C2 symmetric twist conformation, the ring puckering in (I) is a linear combination of 76.5% of the C2 symmetric twist and 23.5% of the Cs symmetric envelope conformation (Evans & Boeyens, 1989). The C2 axis in the room-temperature structure passes through the midpoint of the C—C bond. In (I) at 110 K this midpoint is shifted significantly away from this axis.
The largest change between the room-temperature and the low-temperature phase is in the sulfate anion, which is heavily disordered in P31c and well ordered in P3. In P3 one S—O bond of the sulfate tetrahedron is on a threefold axis, while the Zn—N bonds of the Zn(ethylenediamine)3 octahedron form angles of 55.28 (3) and 121.00 (3)° with the threefold axis, respectively. The ordering of the sulfate now also allows a reliable description of the hydrogen-bonding interactions (Table 2), which was difficult in the room-temperature phase. All four N—H hydrogen atoms act as hydrogen-bond donors with sulfate O atoms as acceptors. Each of the two independent O atoms is an acceptor of three hydrogen bonds. Overall, this is an infinite three-dimensional hydrogen-bonding network.
The solid–solid phase transition temperature for the copper complex, (II), is given in the literature as 180 K (Bertini et al., 1977). A low-temperature crystal structure of the copper complex, (II), has been reported (Bertini et al., 1979) based on X-ray data from a diffractometer with a point detector. The authors found a single crystal with unit-cell parameters similar to the trigonal room-temperature cell, but the space-group symmetry was determined as triclinic P1. The metal complex and the sulfate anion are on general positions (C1 symmetry) and three sulfate O atoms were refined with a disorder model. The coordination octahedron appeared to be compressed. This is in contrast to a [Cu(en)3]Cl2 complex [en = ?] described in the same paper, which has the expected elongated octahedra. The sulfate and the chloride complexes have similar g values in low-temperature ESR [ESR = ?] spectra, which is an indication of a similar geometry of the complexes and contradicts the compression of the octahedron. The presence of three signals in single-crystal ESR spectra would require the presence of three triclinic unit cells, which were not detected in the X-ray diffraction experiment. A contradiction with the compressed geometry of the Cu complex was later also found with XAFS [X-ray absorption fine structure?] (Villain et al., 1997), where equatorial distances of 2.06 and 2.04 Å were determined at room temperature and 10 K, respectively, and axial distances of 2.28 and 2.34 Å.
In our redetermination of (II) at 110 (2) K the indexing program DIRAX (Duisenberg, 1992) finds a C-centred unit cell with a = 17.6371 (9), b = 30.7843 (12), c = 12.9731 (6) Å, α = 89.976 (2), β = 132.408 (2), γ = 90.076 (2)° (based on 805 reflections between θ = 4.12 and θ = 29.87°). This cell is a very rough approximation because it ignores the splitting of the reflections. Additionally this cell predicts too many reflections in some reciprocal planes and too few in others (Fig. 2).
To cover all reflections and to take reflection splitting into account, a triclinic subcell must be chosen together with suitable twin operations. The corresponding cell parameters are a = 8.875, b = 12.972, c = 13.066 Å, α = 71.96, β = 70.15, γ = 70.46°. The volume of this cell is twice the trigonal room-temperature cell. For a better comparison with the room-temperature cell we decided to transform the triclinic P-cell into a non-standard triclinic A-cell (Table 6). Based on this A-centred lattice, 120° rotations about the c axis are selected as twin operations resulting in three twin domains (`Drilling'). This way all reflections are indexed and because of small deviations from exact hexagonal angles the splitting of the reflections can also be explained. Unfortunately, the refinement of the Drilling still gives unsatisfactory results. The addition of twofold rotations perpendicular to the c axis as twin operations results in six twin domains. The inclusion of the six domains into the refinement improves results, but the twin fraction of one domain remains zero. Therefore the final intensity integration with Eval14 (Duisenberg et al., 2003) and the final SHELXL (Sheldrick, 2008b) refinements are based on five twin domains. The corresponding orientation matrices are given in the cif file and the refined batch scale factors were 0.402 (3), 0.0191 (7), 0.3395 (18), 0.1480 (18) and 0.0913 (18). A coset decomposition of 6/mmm with respect to -1 results in 12 cosets and thus 12 potential twin operations. Only five of these are necessary for a satisfactory refinement.
The asymmetric unit of (II) consists of two independent formula units and all cations and anions are on general positions with C1 symmetry (Fig. 3). The two independent cations are related by an approximate threefold rotation about the c axis and their geometries are very similar. A quaternion fit (Mackay, 1984) based on all non-H atoms is shown in Fig. 4. Each cation has four equatorial short [2.045 (3)–2.073 (3) Å] and two axial long Cu—N distances [2.373 (3)–2.396 (4) Å], respectively (Table 3). The equatorial distances are very similar to those in the polymeric [Cu(SO4)(C2H8N2)2]n (Lutz et al., 2010), which has Cu—N distances of 2.0173 (8) and 2.0226 (8) Å. The Jahn–Teller effect, which can at room temperature only be recognized in enlarged anisotropic displacement parameters (Cullen & Lingafelter, 1970), is at 110 (2) K clearly visible in the different Cu—N bond lengths. Here, none of the Cu—N bonds in (II) fails to the Hirshfeld rigid bond test (Hirshfeld, 1976) with more than 5σ.
Despite the absence of symmetry, all four equatorial N atoms still form a plane including the Cu atom [the maximal deviation from the least-squares plane is 0.086 (4) Å]. The N—Cu—N bite angles are smaller than the ideal octahedral 90° [79.64 (12)–83.99 (14)°] and the trans-angles consequently deviate from linearity [166.73 (12)–174.13 (12)°]. The six independent five-membered chelate rings are mainly in twist conformation. If they are considered as a linear combination of the twist and the envelope conformations, the contribution of the envelope conformation ranges from 0.0 to 37.9%.
In contrast to the room-temperature phase the two independent sulfate anions in (II) at 110 K are ordered, but have a different orientation with respect to the cell axes (Fig. 5). In fact, the crystal structure has pseudo-translational symmetry, which is only broken by the O atoms of the sulfate. The ADDSYM routine of PLATON (Spek, 2009) finds an 83% fit for a halving of the c axis. If the O atoms are left out of the analysis, a 100% fit is found. In the standard P-setting of the space group this would correspond to a pseudo I-centring. Despite this pseudo-symmetry no correlation matrix elements for coordinates or displacement parameters are larger than 0.76 in the least-squares refinement. ADDSYM does not indicate the presence of monoclinic or orthorhombic symmetry, while the cell parameters can be transformed to a pseudo-orthorhombic I-cell with angles close to 90°. Merging of the calculated structure factors according to monoclinic or orthorhombic symmetries results in Rint values larger than 0.34.
As the sulfate is ordered in (II), a detailed analysis of the hydrogen bonding is now possible (Table 4). While in the room-temperature phase there are only two independent donor H atoms, (II) at 110 K has 24 independent donor H atoms. There is large variation in the donor—acceptor distances between 2.900 (5) and 3.552 (5) Å. The longest distances belong to the bifurcated hydrogen bonds at H2N and H22N. The hydrogen bonds lead to a densely packed three-dimensional network with a packing index of 76.0% (Kitajgorodskij, 1973).