
The title 2:1 complex of 3-nitrophenol (MNP) and 4,4′-bipyridyl
N,
N′-dioxide (DPNO), 2C
6H
5NO
3·C
10H
8N
2O
2 or 2MNP·DPNO, crystallizes as a centrosymmetric three-component adduct with a dihedral angle of 59.40 (8)° between the planes of the benzene rings of MNP and DPNO (the DPNO moiety lies across a crystallographic inversion centre located at the mid-point of the C—C bond linking its aromatic rings). The complex owes its formation to O—H

O hydrogen bonds [O

O = 2.605 (3) Å]. Molecules are linked by intermolecular C—H

O and C—H

N interactions forming
R21(6) and
R22(10) rings, and
R66(34) and
R44(26) macro-rings, all of which are aligned along the [
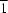
01] direction, and
R22(10) and
R21(7) rings aligned along the [010] direction. The combination of chains of rings along the [
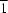
01] and [010] directions generates the three-dimensional structure. A total of 27 systems containing the DNPO molecule and forming molecular complexes of an organic nature were analysed and compared with the structural characteristics of the dioxide reported here. The N—O distance [1.325 (2) Å] depends not only on the interactions involving the O atom at the N—O group, but also on the structural ordering and additional three-dimensional interactions in the crystal structure. A density functional theory (DFT) optimized structure at the B3LYP/6-311G(d,p) level is compared with the molecular structure in the solid state.
Supporting information
CCDC reference: 790651
Reagents and solvents for the synthesis were purchased from Aldrich and were
used without additional purification. Pale-yellow single crystals of the title
molecular complex, (I), suitable for X-ray analysis were obtained by slow
evaporation of an equimolar solution of MNP and DPNO in acetonitrile. The
crystals of the MNP–DPNO complex have a melting point of 589 (1) K.
Atom H31 was located in a difference map and its coordinates were refined
freely. All other H atoms were located in difference maps and then treated as
riding atoms, with C—H = 0.93 Å and Uiso(H) = 1.2Ueq(C).
Data collection: MSC/AFC Diffractometer Control Software
(Molecular Structure Corporation, 1993); cell refinement: MSC/AFC Diffractometer Control Software
(Molecular Structure Corporation, 1993); data reduction: TEXSAN (Molecular Structure Corporation, 1995); program(s) used to solve structure: SHELXS97 (Sheldrick, 2008); program(s) used to refine structure: SHELXL97 (Sheldrick, 2008); molecular graphics: ORTEP-3 (Farrugia, 1997) and Mercury (Macrae et al.,
2008); software used to prepare material for publication: PARST95 (Nardelli, 1995).
3-Nitrophenol–4,4'-bipyridyl
N,
N'-dioxide (2/1)
top
Crystal data top
| 2C6H5NO3·C10H8N2O2 | F(000) = 484 |
| Mr = 466.4 | Dx = 1.403 Mg m−3 |
| Monoclinic, P21/c | Mo Kα radiation, λ = 0.71073 Å |
| Hall symbol: -P 2ybc | Cell parameters from 25 reflections |
| a = 10.5167 (14) Å | θ = 17.2–19.6° |
| b = 5.9729 (13) Å | µ = 0.11 mm−1 |
| c = 18.2602 (10) Å | T = 291 K |
| β = 105.700 (7)° | Prism, yellow |
| V = 1104.2 (3) Å3 | 0.32 × 0.19 × 0.15 mm |
| Z = 2 | |
Data collection top
Rigaku AFC-7S
diffractometer | Rint = 0.016 |
| Radiation source: fine-focus sealed tube | θmax = 25.0°, θmin = 2.0° |
| Graphite monochromator | h = 0→12 |
| ω/2θ scans | k = 0→7 |
| 2052 measured reflections | l = −21→20 |
| 1937 independent reflections | 2 standard reflections every 150 min |
| 1243 reflections with I > 2σ(I) | intensity decay: none |
Refinement top
| Refinement on F2 | Primary atom site location: structure-invariant direct methods |
| Least-squares matrix: full | Secondary atom site location: difference Fourier map |
| R[F2 > 2σ(F2)] = 0.048 | Hydrogen site location: inferred from neighbouring sites |
| wR(F2) = 0.130 | H atoms treated by a mixture of independent and constrained refinement |
| S = 1.01 | w = 1/[σ2(Fo2) + (0.068P)2 + 0.1527P]
where P = (Fo2 + 2Fc2)/3 |
| 1939 reflections | (Δ/σ)max < 0.001 |
| 158 parameters | Δρmax = 0.13 e Å−3 |
| 0 restraints | Δρmin = −0.21 e Å−3 |
Crystal data top
| 2C6H5NO3·C10H8N2O2 | V = 1104.2 (3) Å3 |
| Mr = 466.4 | Z = 2 |
| Monoclinic, P21/c | Mo Kα radiation |
| a = 10.5167 (14) Å | µ = 0.11 mm−1 |
| b = 5.9729 (13) Å | T = 291 K |
| c = 18.2602 (10) Å | 0.32 × 0.19 × 0.15 mm |
| β = 105.700 (7)° | |
Data collection top
Rigaku AFC-7S
diffractometer | Rint = 0.016 |
| 2052 measured reflections | 2 standard reflections every 150 min |
| 1937 independent reflections | intensity decay: none |
| 1243 reflections with I > 2σ(I) | |
Refinement top
| R[F2 > 2σ(F2)] = 0.048 | 0 restraints |
| wR(F2) = 0.130 | H atoms treated by a mixture of independent and constrained refinement |
| S = 1.01 | Δρmax = 0.13 e Å−3 |
| 1939 reflections | Δρmin = −0.21 e Å−3 |
| 158 parameters | |
Special details top
Geometry. All esds (except the esd in the dihedral angle between two l.s. planes) are
estimated using the full covariance matrix. The cell esds are taken into
account individually in the estimation of esds in distances, angles and
torsion angles; correlations between esds in cell parameters are only used
when they are defined by crystal symmetry. An approximate (isotropic)
treatment of cell esds is used for estimating esds involving l.s. planes. |
Refinement. Refinement of F2 against ALL reflections. The weighted R-factor
wR and goodness of fit S are based on F2, conventional
R-factors R are based on F, with F set to zero for
negative F2. The threshold expression of F2 >
σ(F2) is used only for calculating R-factors(gt) etc. and is
not relevant to the choice of reflections for refinement. R-factors
based on F2 are statistically about twice as large as those based on
F, and R- factors based on ALL data will be even larger. |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2) top| | x | y | z | Uiso*/Ueq | |
| O1 | 0.2358 (2) | 0.5744 (3) | 0.54388 (11) | 0.0776 (6) | |
| O2 | 0.3763 (3) | 0.7214 (5) | 0.49546 (15) | 0.1268 (11) | |
| O3 | 0.2511 (2) | 1.0219 (3) | 0.77541 (10) | 0.0646 (5) | |
| H31 | 0.200 (3) | 0.888 (6) | 0.7740 (17) | 0.094 (11)* | |
| N1 | 0.3180 (2) | 0.7149 (4) | 0.54378 (11) | 0.0616 (6) | |
| C1 | 0.3452 (2) | 0.8859 (4) | 0.60354 (12) | 0.0485 (6) | |
| C2 | 0.4253 (3) | 1.0641 (5) | 0.59824 (15) | 0.0654 (8) | |
| H2 | 0.4648 | 1.0742 | 0.5585 | 0.078* | |
| C3 | 0.4449 (3) | 1.2270 (5) | 0.65351 (15) | 0.0681 (8) | |
| H3 | 0.4987 | 1.3490 | 0.6513 | 0.082* | |
| C4 | 0.3861 (3) | 1.2114 (4) | 0.71190 (14) | 0.0576 (7) | |
| H4 | 0.3998 | 1.3234 | 0.7486 | 0.069* | |
| C5 | 0.3067 (2) | 1.0306 (4) | 0.71658 (13) | 0.0481 (6) | |
| C6 | 0.2857 (2) | 0.8647 (4) | 0.66170 (12) | 0.0468 (6) | |
| H6 | 0.2327 | 0.7418 | 0.6641 | 0.056* | |
| O4 | 0.11874 (19) | 0.6496 (3) | 0.76650 (8) | 0.0649 (6) | |
| N2 | 0.0858 (2) | 0.6079 (3) | 0.83024 (10) | 0.0488 (5) | |
| C7 | 0.0090 (3) | 0.7510 (4) | 0.85444 (13) | 0.0529 (6) | |
| H7 | −0.0219 | 0.8791 | 0.8262 | 0.064* | |
| C8 | −0.0249 (2) | 0.7109 (4) | 0.92079 (12) | 0.0508 (6) | |
| H8 | −0.0782 | 0.8133 | 0.9370 | 0.061* | |
| C9 | 0.0181 (2) | 0.5226 (4) | 0.96382 (11) | 0.0391 (5) | |
| C10 | 0.0962 (2) | 0.3773 (4) | 0.93591 (13) | 0.0539 (7) | |
| H10 | 0.1269 | 0.2467 | 0.9626 | 0.065* | |
| C11 | 0.1290 (3) | 0.4219 (4) | 0.86998 (14) | 0.0599 (7) | |
| H11 | 0.1819 | 0.3219 | 0.8525 | 0.072* | |
Atomic displacement parameters (Å2) top| | U11 | U22 | U33 | U12 | U13 | U23 |
| O1 | 0.0987 (15) | 0.0666 (13) | 0.0750 (13) | −0.0275 (12) | 0.0361 (12) | −0.0151 (11) |
| O2 | 0.178 (2) | 0.133 (2) | 0.1113 (17) | −0.068 (2) | 0.1101 (19) | −0.0521 (17) |
| O3 | 0.0838 (13) | 0.0615 (12) | 0.0604 (11) | −0.0068 (11) | 0.0398 (10) | −0.0075 (9) |
| N1 | 0.0739 (15) | 0.0643 (15) | 0.0527 (12) | −0.0106 (13) | 0.0277 (12) | −0.0022 (11) |
| C1 | 0.0504 (14) | 0.0520 (14) | 0.0446 (12) | −0.0063 (12) | 0.0155 (11) | 0.0005 (12) |
| C2 | 0.0694 (18) | 0.077 (2) | 0.0570 (15) | −0.0208 (16) | 0.0289 (14) | 0.0034 (15) |
| C3 | 0.075 (2) | 0.0653 (18) | 0.0644 (17) | −0.0281 (16) | 0.0202 (15) | −0.0012 (15) |
| C4 | 0.0635 (16) | 0.0525 (16) | 0.0547 (15) | −0.0090 (13) | 0.0125 (13) | −0.0036 (12) |
| C5 | 0.0514 (14) | 0.0474 (14) | 0.0475 (13) | 0.0027 (12) | 0.0166 (11) | 0.0033 (12) |
| C6 | 0.0475 (13) | 0.0455 (13) | 0.0509 (13) | −0.0022 (11) | 0.0190 (11) | 0.0053 (12) |
| O4 | 0.1024 (14) | 0.0641 (12) | 0.0421 (9) | −0.0149 (10) | 0.0435 (9) | −0.0035 (8) |
| N2 | 0.0684 (13) | 0.0489 (12) | 0.0360 (10) | −0.0094 (11) | 0.0261 (10) | −0.0032 (9) |
| C7 | 0.0715 (17) | 0.0474 (14) | 0.0451 (13) | 0.0065 (13) | 0.0245 (12) | 0.0095 (12) |
| C8 | 0.0646 (16) | 0.0531 (15) | 0.0415 (12) | 0.0123 (13) | 0.0262 (12) | 0.0054 (12) |
| C9 | 0.0459 (12) | 0.0406 (12) | 0.0325 (10) | −0.0026 (10) | 0.0132 (9) | −0.0025 (10) |
| C10 | 0.0785 (18) | 0.0466 (14) | 0.0451 (13) | 0.0101 (13) | 0.0315 (13) | 0.0055 (12) |
| C11 | 0.0879 (19) | 0.0504 (15) | 0.0539 (14) | 0.0100 (14) | 0.0408 (14) | 0.0006 (13) |
Geometric parameters (Å, º) top
| O1—N1 | 1.205 (3) | C6—H6 | 0.9300 |
| O2—N1 | 1.204 (3) | O4—N2 | 1.325 (2) |
| O3—C5 | 1.356 (3) | N2—C7 | 1.331 (3) |
| O3—H31 | 0.96 (3) | N2—C11 | 1.337 (3) |
| N1—C1 | 1.465 (3) | C7—C8 | 1.374 (3) |
| C1—C2 | 1.377 (3) | C7—H7 | 0.9300 |
| C1—C6 | 1.377 (3) | C8—C9 | 1.376 (3) |
| C2—C3 | 1.376 (4) | C8—H8 | 0.9300 |
| C2—H2 | 0.9300 | C9—C10 | 1.383 (3) |
| C3—C4 | 1.373 (3) | C9—C9i | 1.496 (4) |
| C3—H3 | 0.9300 | C10—C11 | 1.365 (3) |
| C4—C5 | 1.382 (3) | C10—H10 | 0.9300 |
| C4—H4 | 0.9300 | C11—H11 | 0.9300 |
| C5—C6 | 1.384 (3) | | |
| | | |
| C5—O3—H31 | 111.7 (18) | C1—C6—H6 | 120.8 |
| O2—N1—O1 | 122.1 (2) | C5—C6—H6 | 120.8 |
| O2—N1—C1 | 119.2 (2) | O4—N2—C7 | 119.8 (2) |
| O1—N1—C1 | 118.7 (2) | O4—N2—C11 | 120.1 (2) |
| C2—C1—C6 | 122.7 (2) | C7—N2—C11 | 120.05 (19) |
| C2—C1—N1 | 119.1 (2) | N2—C7—C8 | 120.4 (2) |
| C6—C1—N1 | 118.2 (2) | N2—C7—H7 | 119.8 |
| C3—C2—C1 | 117.9 (2) | C8—C7—H7 | 119.8 |
| C3—C2—H2 | 121.0 | C7—C8—C9 | 121.5 (2) |
| C1—C2—H2 | 121.0 | C7—C8—H8 | 119.3 |
| C4—C3—C2 | 120.8 (3) | C9—C8—H8 | 119.3 |
| C4—C3—H3 | 119.6 | C8—C9—C10 | 116.02 (19) |
| C2—C3—H3 | 119.6 | C8—C9—C9i | 121.9 (2) |
| C3—C4—C5 | 120.5 (2) | C10—C9—C9i | 122.1 (2) |
| C3—C4—H4 | 119.7 | C11—C10—C9 | 121.3 (2) |
| C5—C4—H4 | 119.7 | C11—C10—H10 | 119.4 |
| O3—C5—C4 | 118.4 (2) | C9—C10—H10 | 119.4 |
| O3—C5—C6 | 121.9 (2) | N2—C11—C10 | 120.7 (2) |
| C4—C5—C6 | 119.7 (2) | N2—C11—H11 | 119.6 |
| C1—C6—C5 | 118.4 (2) | C10—C11—H11 | 119.6 |
| | | |
| O2—N1—C1—C2 | 7.4 (4) | O3—C5—C6—C1 | 179.8 (2) |
| O1—N1—C1—C2 | −171.2 (2) | C4—C5—C6—C1 | −0.1 (3) |
| O2—N1—C1—C6 | −174.7 (3) | O4—N2—C7—C8 | −179.4 (2) |
| O1—N1—C1—C6 | 6.7 (4) | C11—N2—C7—C8 | 0.9 (4) |
| C6—C1—C2—C3 | −0.4 (4) | N2—C7—C8—C9 | −0.4 (4) |
| N1—C1—C2—C3 | 177.4 (2) | C7—C8—C9—C10 | −0.4 (4) |
| C1—C2—C3—C4 | −0.1 (4) | C7—C8—C9—C9i | 180.0 (3) |
| C2—C3—C4—C5 | 0.5 (4) | C8—C9—C10—C11 | 0.8 (4) |
| C3—C4—C5—O3 | 179.7 (2) | C9i—C9—C10—C11 | −179.6 (3) |
| C3—C4—C5—C6 | −0.4 (4) | O4—N2—C11—C10 | 179.8 (2) |
| C2—C1—C6—C5 | 0.5 (4) | C7—N2—C11—C10 | −0.6 (4) |
| N1—C1—C6—C5 | −177.3 (2) | C9—C10—C11—N2 | −0.3 (4) |
| Symmetry code: (i) −x, −y+1, −z+2. |
Hydrogen-bond geometry (Å, º) top
| D—H···A | D—H | H···A | D···A | D—H···A |
| O3—H31···O4 | 0.96 (3) | 1.65 (3) | 2.605 (3) | 175 (3) |
| O3—H31···N2 | 0.96 (3) | 2.44 (3) | 3.329 (3) | 154 (2) |
| C8—H8···O1ii | 0.93 | 2.37 | 3.290 (3) | 170 |
| C7—H7···O4ii | 0.93 | 2.36 | 3.278 (3) | 168 |
| C2—H2···O2iii | 0.93 | 2.48 | 3.295 (3) | 146 |
| C6—H6···O4 | 0.93 | 2.54 | 3.196 (3) | 128 |
| C10—H10···O1iv | 0.93 | 2.50 | 3.428 (3) | 172 |
| C11—H11···O3v | 0.93 | 2.51 | 3.397 (3) | 161 |
| Symmetry codes: (ii) −x, y+1/2, −z+3/2; (iii) −x+1, −y+2, −z+1; (iv) x, −y+1/2, z+1/2; (v) x, y−1, z. |
Experimental details
| Crystal data |
| Chemical formula | 2C6H5NO3·C10H8N2O2 |
| Mr | 466.4 |
| Crystal system, space group | Monoclinic, P21/c |
| Temperature (K) | 291 |
| a, b, c (Å) | 10.5167 (14), 5.9729 (13), 18.2602 (10) |
| β (°) | 105.700 (7) |
| V (Å3) | 1104.2 (3) |
| Z | 2 |
| Radiation type | Mo Kα |
| µ (mm−1) | 0.11 |
| Crystal size (mm) | 0.32 × 0.19 × 0.15 |
| |
| Data collection |
| Diffractometer | Rigaku AFC-7S
diffractometer |
| Absorption correction | – |
No. of measured, independent and
observed [I > 2σ(I)] reflections | 2052, 1937, 1243 |
| Rint | 0.016 |
| (sin θ/λ)max (Å−1) | 0.595 |
| |
| Refinement |
| R[F2 > 2σ(F2)], wR(F2), S | 0.048, 0.130, 1.01 |
| No. of reflections | 1939 |
| No. of parameters | 158 |
| H-atom treatment | H atoms treated by a mixture of independent and constrained refinement |
| Δρmax, Δρmin (e Å−3) | 0.13, −0.21 |
Hydrogen-bond geometry (Å, º) top
| D—H···A | D—H | H···A | D···A | D—H···A |
| O3—H31···O4 | 0.96 (3) | 1.65 (3) | 2.605 (3) | 175 (3) |
| O3—H31···N2 | 0.96 (3) | 2.44 (3) | 3.329 (3) | 154 (2) |
| C8—H8···O1i | 0.93 | 2.37 | 3.290 (3) | 170.1 |
| C7—H7···O4i | 0.93 | 2.36 | 3.278 (3) | 167.8 |
| C2—H2···O2ii | 0.93 | 2.48 | 3.295 (3) | 146.4 |
| C6—H6···O4 | 0.93 | 2.54 | 3.196 (3) | 127.9 |
| C10—H10···O1iii | 0.93 | 2.50 | 3.428 (3) | 172.3 |
| C11—H11···O3iv | 0.93 | 2.51 | 3.397 (3) | 160.7 |
| Symmetry codes: (i) −x, y+1/2, −z+3/2; (ii) −x+1, −y+2, −z+1; (iii) x, −y+1/2, z+1/2; (iv) x, y−1, z. |
 O hydrogen bonds [O
O hydrogen bonds [O O = 2.605 (3) Å]. Molecules are linked by intermolecular C—H
O = 2.605 (3) Å]. Molecules are linked by intermolecular C—H O and C—H
O and C—H N interactions forming R21(6) and R22(10) rings, and R66(34) and R44(26) macro-rings, all of which are aligned along the [
N interactions forming R21(6) and R22(10) rings, and R66(34) and R44(26) macro-rings, all of which are aligned along the [
 journal menu
journal menu








![[Figure 1]](http://journals.iucr.org/c/issues/2010/08/00/gg3235/gg3235fig1thm.gif)
![[Figure 2]](http://journals.iucr.org/c/issues/2010/08/00/gg3235/gg3235fig2thm.gif)
![[Figure 3]](http://journals.iucr.org/c/issues/2010/08/00/gg3235/gg3235fig3thm.gif)




The title compound, 2MNP.DPNO (MNP is 3-nitrophenol and DPNO is 4,4'-bipyridyl N,N'-dioxide), (I), belongs to a series of molecular systems based on 4,4'-bipyridyl N,N'-dioxide with diverse hydrogen-bond donors (Moreno-Fuquen et al., 2003; Lou & Huang, 2007). Crystal engineering with desired functions and unexpected topological architectures is of great interest in the area of solid-state science (Fujita et al., 1995; Hagrman et al., 1999). Much effort has focused on supramolecular network analysis with non-covalent interactions. This interest is motivated by the role played by hydrogen bonds and other weak interactions on molecular recognition (Aruksankunwong et al., 2006) and on the physical, chemical and biological properties of crystal systems (Steiner & Saenger, 1995; Zhang et al., 2008). In this work, we have chosen the 4,4'-bipyridine dioxide and 3-nitrophenol adduct (DPNO+MNP), considering its capability of participating in hydrogen bonding. To complement this analysis, 27 different organic crystalline systems, where the DPNO is involved in the formation of supramolecular networks, have been chosen for comparison [Cambridge Structural Database (CSD), Version 5.29; Allen, 2002].
It is of interest to analyse the behaviour of DPNO in different crystal environments and examine which structural parameters of this molecule are sensitive to the formation of molecular complexes.
A perspective view of (I), showing the atom-numbering scheme, is given in Fig. 1. This 2MNP.DPNO molecular complex owes its formation to an intermolecular hydrogen bond between the O—H group of the MNP molecule and the N—O group of the DPNO molecule, with an O3···O4 distance of 2.605 (3) Å (Fig. 1). The dihedral angle between the planes of the MNP and DPNO rings is 59.40 (8)°. Atom O3 of the MNP molecule is linked to atom N2 of the DPNO molecule, forming an additional weak intermolecular O3—H3···N2 hydrogen bond, with an O3···N2 distance of 3.329 (2) Å and an angle of 154.3 (3)°. This interaction can explain the stability of the title adduct and it is likely that it can fix and orient the DPNO rings in the crystal structure. The distances and bond angles of (I) are similar to those found in free DPNO (Thaimattan et al., 1998) and MNP (Wojcik et al., 2006; Hamzaoui et al., 2007).
The analysis of 27 different organic molecular systems where DPNO is involved allows us to examine the behaviour and structural affinities of this molecule, and these are defined below.
A detailed analysis of the behaviour of the DPNO adduct shows three parameters that are sensitive when it forms molecular complexes with different proton donors: the N—O bond length, the interplanar angle between the rings and the C—C bond length between the rings.
The O atom of the N—O group in DPNO is involved in the formation of several intermolecular interactions, including hydrogen bonding. Atom O4 is linked to hydrogen-bond donors, forming O···H—O, O···H—N and weak O···H—C interactions. The change in bond length of N—O was examined in 78 structures of pyridine N-oxide complexes, and a mean value of 1.293 Å in free N-oxide groups was reported (Eichhorn, 1987). In other N-oxide systems such as 4-nitropyridine N-oxide, the N—O bond is shorter, at 1.260 Å (Eichhorn, 1956). In the series of 27 organic compounds with DPNO, the shorter N—O bond lengths [1.296 (4) and 1.297 (3) Å] are observed in the structure of 9,9'-biacridine N,N'-dioxide (CSD refcode GINSAY; Liu, 2007). In this system, the molecular building blocks are based on bulky acridine N-oxide rings with a T-shaped conformation (torsion angles between the rings are close to 100°). This shows a steric hindrance between the H atoms of adjacent benzene rings, affecting the ability of the N—O group to form multiple hydrogen-bond interactions and thus inhibiting further elongation of the N—O bond. Even so, this structural feature allows the formation of supramolecular chains of R22(18) rings (Etter, 1990) along the [201] direction by weak N···H—C interactions.
The longest N—O bond in this series is in 4,4'-bipyridinium cis,cis-cyclohexane-1,3,5-tricarboxylic acid 4,4'-dipyridine-N,N'-dioxide cyclohexane-1-carboxylate-cis,cis-3,5-dicarboxylic acid dihydrate (CSD refcode JAWWUA; Bhogala et al., 2005), where the DPNO molecule is part of an unusual system, a ternary co-crystal. The O atom of the N—O group in the molecule of DPNO is positioned in different environments in the formation of hydrogen bonds. In the first N—O group of DPNO, the O atom forms hydrogen bonds with two different donor molecules: with the cis,cis-cyclohexane-1,3,5-tricarboxylic acid (CTA) in the bc plane [O···H—O = 2.6306 (16) Å] and with a water molecule in the ab plane [O···H—O = 2.7949 (18) Å]. These interactions affect the N—O bond length [1.338 (2) Å]. In the second N—O group of DPNO, the O atom is linked with only one molecule of CTA [O···H—O = 2.5605 (18) Å], achieving a smaller N—O bond length [1.321 (2) Å]. The O···H—O hydrogen-bond angles are more or less similar in these interactions (~ 168°).
The difference in the N—O bond lengths in these structures depends not only on the interactions formed by the O atom of the N—O group, but also on the structural ordering and additional three-dimensional interactions in the crystal structure. In this case, for the first N—O group of DPNO, the formation of chains of large rings with an R88(50) motif in the bc plane allows the interaction of DPNO, CTA, water and pyridine molecules with each other. In some sections of this chain the presence of local charges is observed. For the second N—O group, molecules of DPNO, CTA and water form R66(42) rings in the ab plane, but these molecules are distant from one another and have no additional interactions between them.
In this series, symmetric stretching of the N—O bonds of DPNO is also observed in some compounds, where there is coplanarity between the rings of the DPNO molecule [CSD refcodes VIGGEY (Babu et al., 2007), OCOMUO (Messina et al., 2001), MEGDIM (Reddy et al., 2006), LICJUD (Lou & Huang, 2007), LAPLEU (Zeng et al., 2005) and HUZCUA (Moreno-Fuquen et al., 2003)]. Thus, in analyzing the behaviour of supramolecular systems that exhibit this property, we can conclude that the environments formed by the hydrogen bonds at both N—O groups of the DPNO molecule are identical. An inversion centre in these compounds confirms this behaviour. Additionally, a dependency is observed that is not very strong, between the C9—C9a and N—O distances. This effect is evident when the dihedral angle between the DPNO rings increases (CSD refcode GINSAY; Liu, 2007).
We next undertook a theoretical study of the adduct. The crystallographic structure parameters of (I) were used as a starting point for the calculations. The DFT method was applied, with the B3LYP hybrid-exchange correlation function (Becke, 1993; Lee et al., 1988) and the 6-311G(d,p) basis set (Bauschlicher & Partridge, 1995), as implemented in GAUSSIAN03 (Frisch et al., 2004). The results are presented in Table S1 in the supplementary material, together with experimental data obtained by X-ray diffraction.
The optimized geometry shows differences with respect to the experimental crystal structure for the N2—C7 and N2—C11 bond lengths (see Table S1). The C9—C9a distance is very close between the two structures. The theoretical study also gives a dihedral angle of 30.3188° between the planes of the DPNO rings and different values for the N2—O4 bond lengths (1.2939 and 1.2655 Å). The reason for the discrepancy between the observed and calculated values should be considered in terms of the effects of crystal packing.
The experimental structure of (I) shows O—H···O hydrogen-bonding interactions, and it also exhibits weak C—H···O and C—H···N intermolecular interactions (see Table 2) (Nardelli, 1995). The molecules of the adduct are linked into a two-dimensional substructure built from O—H···O and C—H···N hydrogen bonds and weak C—H···O interactions, generating a continuous framework structure. The construction of the first substructure is obtained from the cooperation of five different hydrogen bonds and weak interactions that bind to a DPNO molecule. The formation of chains of different rings is observed between DPNO molecules along the [101] direction (Fig. 2). Indeed, for the formation of the first type of rings, interactions between DPNO and MNP molecules are observed. For this, atom O3 in the molecule at (x, y, z) acts simultaneously as hydrogen-bond donor to atoms O4 and N2 in the molecule at (x, y, z). In turn, atom C6 in the molecule at (x, y, z) acts as hydrogen-bond donor to atom O4 in the molecule at (x, y, z), and atom C2 in the molecule at (x, y, z) acts as hydrogen-bond donor to atom O2i in the molecule at (-x + 1, -y + 2, -z + 1). These interactions allow the formation of R21(3), R12(6) and R22(10) rings. An additional interaction, in which atom C11 in the molecule at (x, y, z) acts as hydrogen-bond donor to atom O3ii in the molecule at (x, y, z + 1), allows the formation of a large ring with an R66(34) motif, which includes the previous rings. The formation of the second type of ring relies only on interactions between DPNO molecules. [Original text not clear - please check rephrasing] Thus, atom C7iii in the molecule at (-x, y + 1/2, -z + 3/2) acts as hydrogen-bond donor to atom O4 in the molecule at (x, y, z), so generating by translation an R44(26) centrosymmetric ring (Fig. 2). In the second one-dimensional substructure within the asymmetric unit of (I), atom C8i in the molecule at (-x, y + 1/2, -z + 3/2) and atom C10ii in the molecule at (x, -y + 1/2, z + 1/2) act as hydrogen-bond donors to atom O1 in the molecule at (x, y, z), so forming an R21(7) ring. At the same time, atom C2 at (x, y, z) acts as hydrogen-bond donor to atom O2iii at (-x + 1, -y + 2, -z + 1), thus forming an R22(10) ring. Both rings run along the [010] direction (Fig. 3). The combination of these chains of rings along [101] and [010] is sufficient to generate the three-dimensional structure of (I).
In summary, this crystallographic study has allowed the analysis of 27 systems containing the DPNO molecule that form molecular complexes of an organic nature. The N—O bond distance depends on the angle between the planes of the DPNO rings. A tendency for symmetric stretching of the N—O bond lengths is observed when the rings of the DPNO molecule are coplanar. It is also noted that, as the angle between the planes of the DPNO molecule increases, the N—O bond length decreases.
A DFT calculation on (I) allowed us to find a structure with a dihedral angle of 30.3188° between the planes of the DPNO rings. This situation revealed the presence of two different N—O distances. There is a modest relationship with a decrease in the N—O bond length that leads to an increase in the C9—C9a distance. This behaviour is represented effectively in the GINSAY complex, and repeated in most of the systems analysed. The N—O distance depends not only on the interactions formed by the O atom at the N—O group, but also on the structural ordering and additional three-dimensional interactions in the crystal structure.