Buy article online - an online subscription or single-article purchase is required to access this article.
The new layered title compound, barium di-μ-hydroxido-di-μ-vanadato-tricobaltate(II), was prepared under low-temperature hydrothermal conditions. Its crystal structure comprises Co
2+ and O
2− ions in the Kagomé geometry. The octahedral Co
3O
6(OH)
2 Kagomé layers, made up of edge-shared CoO
4(OH)
2 octahedra with Co on a site of 2/
m symmetry, alternate along the
c axis with barium vanadate heteropolyhedral layers, in which Ba is on a site of
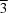 m
m symmetry and V is on a site of 3
m symmetry. All three O atoms and the H atom also occupy special positions: two O atoms and the H atom are on sites with 3
m symmetry and one O atom is on a site with
m symmetry. Ba[Co
3(VO
4)
2(OH)
2] represents the first compound from the four-component BaO–CoO–V
2O
5–H
2O system and its structure is topologically related to the minerals vesignieite, Ba[Cu
3(VO
4)
2(OH)
2], and bayldonite, Pb[Cu
3(AsO
4)
2(OH)
2].
Supporting information
Single crystals of Ba[Co3(VO4)2(OH)2] were obtained as reaction
products from a mixture of Ba(OH)2.H2O (Mallinckrodt 3772, >97%), Co
powder (Merck 1221) and V2O5 (Fluka Chemika 94710, = 98%). The mixture was
transferred into a Teflon vessel and filled to approximately 75% of the inner
volume with distilled water. The pH of the mixture was 12. The Teflon vessel
was enclosed in a stainless steel autoclave, which was then heated under a
three-step heating regime: the autoclave was heated from 293 to 423 K (4 h),
held at 423 K for 120 h, and finally cooled to room temperature within 96 h.
At the end of the reaction, the pH of the solution was 6. The reaction
products were filtered off and washed thoroughly with distilled water.
Ba[Co3(VO4)2(OH)2] crystallized as transparent pale-pink needle-like
crystals up to 0.2 mm in length (yield ca 35%), together with an
uninvestigated powder consisting of dark- and light-brown crusts and
undissolved V2O5 (yield ca 65%).
Studies of several single crystals of Ba[Co3(VO4)2(OH)2] all revealed
the same metrically hexagonal unit cell. A crystal exhibiting sharp reflection
spots was chosen for the data collection. The H atom was located from a
difference Fourier map and refined with the O—H bond distance restrained to
0.82 (2)Å, and with Uiso(H) = 1.5Ueq(O).
Data collection: COLLECT (Nonius, 2002); cell refinement: SCALEPACK (Otwinowski & Minor, 1997); data reduction: DENZO-SMN (Otwinowski et al., 2003); program(s) used to solve structure: SIR97 (Altomare et al., 1999); program(s) used to refine structure: WinGX (Farrugia, 1999) SHELXL97 (Sheldrick, 2008); molecular graphics: ATOMS (Dowty, 2000); software used to prepare material for publication: publCIF (Westrip, 2010).
Barium di-µ-hydroxydo-di-µ-vanadato-tricobaltate(II)
top
Crystal data top
| Ba[Co3(VO4)2(OH)2] | F(000) = 795 |
| Mr = 578.03 | Dx = 4.513 Mg m−3 |
| Rhombohedral, R3m | Mo Kα radiation, λ = 0.71069 Å |
| Hall symbol: -R 3 2" | Cell parameters from 4406 reflections |
| a = 5.9210 (8) Å | θ = 0.4–32.6° |
| c = 21.016 (4) Å | µ = 12.42 mm−1 |
| α = 90° | T = 298 K |
| γ = 120° | Needle-like, light pink |
| V = 638.07 (18) Å3 | 0.07 × 0.06 × 0.04 mm |
| Z = 3 | |
Data collection top
Nonius KappaCCD area-detector
diffractometer | 222 independent reflections |
| Radiation source: fine-focus sealed tube | 215 reflections with I > 2σ(I) |
| Graphite monochromator | Rint = 0.023 |
| ϕ and ω scans | θmax = 27.8°, θmin = 2.9° |
Absorption correction: multi-scan
(Otwinowski et al., 2003) | h = −7→7 |
| Tmin = 0.477, Tmax = 0.637 | k = −7→7 |
| 1909 measured reflections | l = −27→27 |
Refinement top
| Refinement on F2 | Secondary atom site location: difference Fourier map |
| Least-squares matrix: full | Hydrogen site location: inferred from neighbouring sites |
| R[F2 > 2σ(F2)] = 0.018 | Only H-atom coordinates refined |
| wR(F2) = 0.060 | w = 1/[σ2(Fo2) + (0.0326P)2 + 3.6751P]
where P = (Fo2 + 2Fc2)/3 |
| S = 1.33 | (Δ/σ)max < 0.001 |
| 222 reflections | Δρmax = 1.07 e Å−3 |
| 24 parameters | Δρmin = −0.57 e Å−3 |
| 1 restraint | Extinction correction: SHELXL97 (Sheldrick, 2008), Fc*=kFc[1+0.001xFc2λ3/sin(2θ)]-1/4 |
| Primary atom site location: structure-invariant direct methods | Extinction coefficient: 0.0017 (5) |
Crystal data top
| Ba[Co3(VO4)2(OH)2] | V = 638.07 (18) Å3 |
| Mr = 578.03 | Z = 3 |
| Rhombohedral, R3m | Mo Kα radiation |
| a = 5.9210 (8) Å | µ = 12.42 mm−1 |
| c = 21.016 (4) Å | T = 298 K |
| α = 90° | 0.07 × 0.06 × 0.04 mm |
| γ = 120° | |
Data collection top
Nonius KappaCCD area-detector
diffractometer | 222 independent reflections |
Absorption correction: multi-scan
(Otwinowski et al., 2003) | 215 reflections with I > 2σ(I) |
| Tmin = 0.477, Tmax = 0.637 | Rint = 0.023 |
| 1909 measured reflections | |
Refinement top
| R[F2 > 2σ(F2)] = 0.018 | 1 restraint |
| wR(F2) = 0.060 | Only H-atom coordinates refined |
| S = 1.33 | Δρmax = 1.07 e Å−3 |
| 222 reflections | Δρmin = −0.57 e Å−3 |
| 24 parameters | |
Special details top
Geometry. All e.s.d.'s (except the e.s.d. in the dihedral angle between two l.s. planes)
are estimated using the full covariance matrix. The cell e.s.d.'s are taken
into account individually in the estimation of e.s.d.'s in distances, angles
and torsion angles; correlations between e.s.d.'s in cell parameters are only
used when they are defined by crystal symmetry. An approximate (isotropic)
treatment of cell e.s.d.'s is used for estimating e.s.d.'s involving l.s.
planes. |
Refinement. Several crystals of the title compound were selected for single-crystal studies
with a Nonius KappaCCD single-crystal four-circle diffractometer [Mo tube,
graphite monochromator, CCD detector frame size: 621×576 pixels (binned
mode)], equipped with a 300 µm diameter capillary-optics collimator. A
complete sphere of reciprocal space (ϕ and ω scans) was measured at room
temperature for a suitable crystal. The intensity data were processed with the
Nonius program suite DENZO-SMN (Otwinowski & Minor, 1997) and corrected for
Lorentz, polarization and background effects by the multi-scan method
(Otwinowski & Minor, 1997; Otwinowski et al., 2003) for absorption. The data
were processed with WinGX (Farrugia, 1999) and SHELXL97 (Sheldrick, 2008). Refinement of F2 against ALL reflections. The weighted R-factor
wR and goodness of fit S are based on F2, conventional
R-factors R are based on F, with F set to zero for
negative F2. The threshold expression of F2 >
σ(F2) is used only for calculating R-factors(gt) etc.
and is not relevant to the choice of reflections for refinement.
R-factors based on F2 are statistically about twice as large
as those based on F, and R-factors based on ALL data will be
even larger. |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2) top| | x | y | z | Uiso*/Ueq | |
| Ba1 | 0.6667 | 0.3333 | 0.8333 | 0.0213 (3) | |
| Co1 | 0.8333 | 0.6667 | 0.6667 | 0.0108 (3) | |
| V1 | 0.3333 | −0.3333 | 0.75329 (5) | 0.0072 (3) | |
| O1 | 0.3333 | −0.3333 | 0.8323 (3) | 0.0281 (14) | |
| O2 | 0.4955 (3) | 0.5045 (3) | 0.72806 (14) | 0.0110 (6) | |
| O3 | 1.0000 | 1.0000 | 0.71082 (19) | 0.0053 (9) | |
| H1 | 1.0000 | 1.0000 | 0.7494 (10) | 0.008* | |
Atomic displacement parameters (Å2) top| | U11 | U22 | U33 | U12 | U13 | U23 |
| Ba1 | 0.0276 (3) | 0.0276 (3) | 0.0086 (4) | 0.01379 (17) | 0.000 | 0.000 |
| Co1 | 0.0116 (4) | 0.0090 (4) | 0.0111 (4) | 0.0045 (2) | −0.00055 (13) | −0.0011 (3) |
| V1 | 0.0078 (4) | 0.0078 (4) | 0.0060 (5) | 0.0039 (2) | 0.000 | 0.000 |
| O1 | 0.038 (2) | 0.038 (2) | 0.008 (2) | 0.0191 (11) | 0.000 | 0.000 |
| O2 | 0.0104 (10) | 0.0104 (10) | 0.0128 (13) | 0.0055 (10) | 0.0005 (5) | −0.0005 (5) |
| O3 | 0.0062 (13) | 0.0062 (13) | 0.004 (2) | 0.0031 (7) | 0.000 | 0.000 |
Geometric parameters (Å, º) top
| Ba1—O2i | 2.824 (3) | Co1—O3 | 1.9449 (19) |
| Ba1—O2ii | 2.824 (3) | Co1—O2x | 2.161 (2) |
| Ba1—O2iii | 2.824 (3) | Co1—O2xi | 2.161 (2) |
| Ba1—O2iv | 2.824 (3) | Co1—O2 | 2.161 (2) |
| Ba1—O2v | 2.824 (3) | Co1—O2v | 2.161 (2) |
| Ba1—O2 | 2.824 (3) | Co1—Co1iv | 2.9605 (4) |
| Ba1—O1iii | 3.4186 (5) | V1—O1 | 1.661 (6) |
| Ba1—O1vi | 3.4186 (5) | V1—O2xii | 1.745 (3) |
| Ba1—O1 | 3.4186 (5) | V1—O2iv | 1.745 (3) |
| Ba1—O1vii | 3.4186 (5) | V1—O2xiii | 1.745 (3) |
| Ba1—O1viii | 3.4186 (5) | O3—O1iii | 2.596 (7) |
| Ba1—O1ix | 3.4186 (5) | O3—H1 | 0.81 (2) |
| Co1—O3x | 1.9449 (19) | | |
| | | |
| O2i—Ba1—O2ii | 65.14 (9) | O3—Co1—O2v | 93.05 (9) |
| O2i—Ba1—O2iii | 65.14 (9) | O2x—Co1—O2v | 90.54 (15) |
| O2ii—Ba1—O2iii | 65.14 (9) | O2xi—Co1—O2v | 179.998 (2) |
| O2i—Ba1—O2iv | 179.999 (1) | O2—Co1—O2v | 89.46 (15) |
| O2ii—Ba1—O2iv | 114.86 (10) | O3x—Co1—Co1xiv | 139.56 (7) |
| O2iii—Ba1—O2iv | 114.86 (9) | O3—Co1—Co1xiv | 40.44 (7) |
| O2i—Ba1—O2v | 114.86 (10) | O2x—Co1—Co1xiv | 46.75 (5) |
| O2ii—Ba1—O2v | 180.00 (10) | O2xi—Co1—Co1xiv | 91.07 (7) |
| O2iii—Ba1—O2v | 114.86 (9) | O2—Co1—Co1xiv | 133.25 (5) |
| O2iv—Ba1—O2v | 65.14 (9) | O2v—Co1—Co1xiv | 88.93 (6) |
| O2i—Ba1—O2 | 114.86 (9) | O3x—Co1—Co1xv | 139.56 (7) |
| O2ii—Ba1—O2 | 114.86 (9) | O3—Co1—Co1xv | 40.44 (7) |
| O2iii—Ba1—O2 | 180.0 | O2x—Co1—Co1xv | 91.07 (6) |
| O2iv—Ba1—O2 | 65.14 (9) | O2xi—Co1—Co1xv | 46.75 (5) |
| O2v—Ba1—O2 | 65.14 (9) | O2—Co1—Co1xv | 88.93 (6) |
| O2i—Ba1—O1iii | 51.20 (11) | O2v—Co1—Co1xv | 133.25 (5) |
| O2ii—Ba1—O1iii | 107.81 (8) | Co1xiv—Co1—Co1xv | 60.0 |
| O2iii—Ba1—O1iii | 107.81 (8) | O1—V1—O2xii | 107.69 (10) |
| O2iv—Ba1—O1iii | 128.80 (11) | O1—V1—O2iv | 107.69 (10) |
| O2v—Ba1—O1iii | 72.19 (8) | O2xii—V1—O2iv | 111.20 (9) |
| O2—Ba1—O1iii | 72.19 (8) | O1—V1—O2xiii | 107.69 (10) |
| O2i—Ba1—O1vi | 107.81 (8) | O2xii—V1—O2xiii | 111.20 (9) |
| O2ii—Ba1—O1vi | 51.20 (11) | O2iv—V1—O2xiii | 111.20 (9) |
| O2iii—Ba1—O1vi | 107.81 (8) | O1—V1—Co1xii | 121.589 (17) |
| O2iv—Ba1—O1vi | 72.19 (8) | O2xii—V1—Co1xii | 30.46 (5) |
| O2v—Ba1—O1vi | 128.80 (11) | O2iv—V1—Co1xii | 122.94 (9) |
| O2—Ba1—O1vi | 72.19 (8) | O2xiii—V1—Co1xii | 80.84 (5) |
| O1iii—Ba1—O1vi | 119.997 (2) | V1—O1—Ba1 | 90.36 (9) |
| O2i—Ba1—O1 | 128.80 (11) | V1—O1—Ba1xvi | 90.36 (9) |
| O2ii—Ba1—O1 | 72.19 (8) | Ba1—O1—Ba1xvi | 119.996 (2) |
| O2iii—Ba1—O1 | 72.19 (8) | V1—O1—Ba1xiii | 90.36 (9) |
| O2iv—Ba1—O1 | 51.20 (11) | Ba1—O1—Ba1xiii | 119.996 (2) |
| O2v—Ba1—O1 | 107.81 (8) | Ba1xvi—O1—Ba1xiii | 119.996 (2) |
| O2—Ba1—O1 | 107.81 (8) | V1viii—O2—Co1 | 125.37 (10) |
| O1iii—Ba1—O1 | 180.0 | V1viii—O2—Co1iv | 125.37 (10) |
| O1vi—Ba1—O1 | 60.004 (3) | Co1—O2—Co1iv | 86.49 (10) |
| O2i—Ba1—O1vii | 72.19 (8) | V1viii—O2—Ba1 | 110.75 (13) |
| O2ii—Ba1—O1vii | 128.80 (11) | Co1—O2—Ba1 | 102.04 (9) |
| O2iii—Ba1—O1vii | 72.19 (8) | Co1iv—O2—Ba1 | 102.04 (9) |
| O2iv—Ba1—O1vii | 107.81 (8) | V1viii—O2—Co1xvii | 70.84 (6) |
| O2v—Ba1—O1vii | 51.20 (11) | Co1—O2—Co1xvii | 122.49 (11) |
| O2—Ba1—O1vii | 107.81 (8) | Co1iv—O2—Co1xvii | 54.58 (4) |
| O1iii—Ba1—O1vii | 60.004 (3) | Ba1—O2—Co1xvii | 124.25 (4) |
| O1vi—Ba1—O1vii | 180.0 | V1viii—O2—Co1xv | 70.84 (6) |
| O1—Ba1—O1vii | 119.996 (2) | Co1—O2—Co1xv | 54.58 (4) |
| O2i—Ba1—O1viii | 72.19 (8) | Co1iv—O2—Co1xv | 122.49 (11) |
| O2ii—Ba1—O1viii | 72.19 (8) | Ba1—O2—Co1xv | 124.25 (4) |
| O2iii—Ba1—O1viii | 128.80 (11) | Co1xvii—O2—Co1xv | 109.18 (7) |
| O2iv—Ba1—O1viii | 107.81 (8) | Co1—O3—Co1xv | 99.12 (13) |
| O2v—Ba1—O1viii | 107.81 (8) | Co1—O3—Co1xiv | 99.12 (13) |
| O2—Ba1—O1viii | 51.20 (11) | Co1xv—O3—Co1xiv | 99.12 (13) |
| O1iii—Ba1—O1viii | 60.004 (3) | Co1—O3—Ba1viii | 129.65 (4) |
| O1vi—Ba1—O1viii | 60.004 (3) | Co1xv—O3—Ba1viii | 65.48 (6) |
| O1—Ba1—O1viii | 119.996 (3) | Co1xiv—O3—Ba1viii | 129.65 (4) |
| O1vii—Ba1—O1viii | 119.996 (3) | Co1—O3—Ba1vii | 129.65 (4) |
| O2i—Ba1—O1ix | 107.81 (8) | Co1xv—O3—Ba1vii | 129.65 (4) |
| O2ii—Ba1—O1ix | 107.81 (8) | Co1xiv—O3—Ba1vii | 65.48 (6) |
| O2iii—Ba1—O1ix | 51.20 (11) | Ba1viii—O3—Ba1vii | 87.54 (6) |
| O2iv—Ba1—O1ix | 72.19 (8) | Co1—O3—Ba1 | 65.48 (6) |
| O2v—Ba1—O1ix | 72.19 (8) | Co1xv—O3—Ba1 | 129.65 (4) |
| O2—Ba1—O1ix | 128.80 (11) | Co1xiv—O3—Ba1 | 129.65 (4) |
| O1iii—Ba1—O1ix | 119.996 (3) | Ba1viii—O3—Ba1 | 87.54 (6) |
| O1vi—Ba1—O1ix | 119.996 (3) | Ba1vii—O3—Ba1 | 87.54 (6) |
| O1—Ba1—O1ix | 60.004 (2) | Co1—O3—Ba1xviii | 61.50 (10) |
| O1vii—Ba1—O1ix | 60.004 (2) | Co1xv—O3—Ba1xviii | 61.50 (10) |
| O1viii—Ba1—O1ix | 180.0 | Co1xiv—O3—Ba1xviii | 61.50 (10) |
| O3x—Co1—O3 | 179.999 (1) | Ba1viii—O3—Ba1xviii | 126.99 (4) |
| O3x—Co1—O2x | 93.05 (9) | Ba1vii—O3—Ba1xviii | 126.99 (4) |
| O3—Co1—O2x | 86.95 (9) | Ba1—O3—Ba1xviii | 126.99 (4) |
| O3x—Co1—O2xi | 93.05 (9) | Co1—O3—H1 | 118.50 (10) |
| O3—Co1—O2xi | 86.95 (9) | Co1xv—O3—H1 | 118.50 (10) |
| O2x—Co1—O2xi | 89.46 (15) | Co1xiv—O3—H1 | 118.50 (11) |
| O3x—Co1—O2 | 86.95 (9) | Ba1viii—O3—H1 | 53.01 (4) |
| O3—Co1—O2 | 93.05 (9) | Ba1vii—O3—H1 | 53.01 (5) |
| O2x—Co1—O2 | 180.0 | Ba1—O3—H1 | 53.01 (4) |
| O2xi—Co1—O2 | 90.54 (15) | Ba1xviii—O3—H1 | 180.000 (9) |
| O3x—Co1—O2v | 86.95 (9) | | |
| Symmetry codes: (i) y+1/3, −x+y+2/3, −z+5/3; (ii) x−y+1/3, x−1/3, −z+5/3; (iii) −x+4/3, −y+2/3, −z+5/3; (iv) −y+1, x−y, z; (v) −x+y+1, −x+1, z; (vi) −x+1/3, −y−1/3, −z+5/3; (vii) x+1, y+1, z; (viii) x, y+1, z; (ix) −x+4/3, −y−1/3, −z+5/3; (x) −x+5/3, −y+4/3, −z+4/3; (xi) x−y+2/3, x+1/3, −z+4/3; (xii) −x+y, −x, z; (xiii) x, y−1, z; (xiv) −y+2, x−y+1, z; (xv) −x+y+1, −x+2, z; (xvi) x−1, y−1, z; (xvii) −x+y, −x+1, z; (xviii) x+1/3, y+2/3, z−1/3. |
Hydrogen-bond geometry (Å, º) top
| D—H···A | D—H | H···A | D···A | D—H···A |
| O3—H1···O1iii | 0.81 (2) | 1.79 (2) | 2.596 (7) | 180 |
| Symmetry code: (iii) −x+4/3, −y+2/3, −z+5/3. |
Experimental details
| Crystal data |
| Chemical formula | Ba[Co3(VO4)2(OH)2] |
| Mr | 578.03 |
| Crystal system, space group | Rhombohedral, R3m |
| Temperature (K) | 298 |
| a, c (Å) | 5.9210 (8), 21.016 (4) |
| V (Å3) | 638.07 (18) |
| Z | 3 |
| Radiation type | Mo Kα |
| µ (mm−1) | 12.42 |
| Crystal size (mm) | 0.07 × 0.06 × 0.04 |
| |
| Data collection |
| Diffractometer | Nonius KappaCCD area-detector
diffractometer |
| Absorption correction | Multi-scan
(Otwinowski et al., 2003) |
| Tmin, Tmax | 0.477, 0.637 |
No. of measured, independent and
observed [I > 2σ(I)] reflections | 1909, 222, 215 |
| Rint | 0.023 |
| (sin θ/λ)max (Å−1) | 0.656 |
| |
| Refinement |
| R[F2 > 2σ(F2)], wR(F2), S | 0.018, 0.060, 1.33 |
| No. of reflections | 222 |
| No. of parameters | 24 |
| No. of restraints | 1 |
| H-atom treatment | Only H-atom coordinates refined |
| Δρmax, Δρmin (e Å−3) | 1.07, −0.57 |
Selected bond lengths (Å) top| Ba1—O2i | 2.824 (3) | Co1—O3x | 1.9449 (19) |
| Ba1—O2ii | 2.824 (3) | Co1—O3 | 1.9449 (19) |
| Ba1—O2iii | 2.824 (3) | Co1—O2x | 2.161 (2) |
| Ba1—O2iv | 2.824 (3) | Co1—O2xi | 2.161 (2) |
| Ba1—O2v | 2.824 (3) | Co1—O2 | 2.161 (2) |
| Ba1—O2 | 2.824 (3) | Co1—O2v | 2.161 (2) |
| Ba1—O1iii | 3.4186 (5) | Co1—Co1iv | 2.9605 (4) |
| Ba1—O1vi | 3.4186 (5) | V1—O1 | 1.661 (6) |
| Ba1—O1 | 3.4186 (5) | V1—O2xii | 1.745 (3) |
| Ba1—O1vii | 3.4186 (5) | V1—O2iv | 1.745 (3) |
| Ba1—O1viii | 3.4186 (5) | V1—O2xiii | 1.745 (3) |
| Ba1—O1ix | 3.4186 (5) | | |
| Symmetry codes: (i) y+1/3, −x+y+2/3, −z+5/3; (ii) x−y+1/3, x−1/3, −z+5/3; (iii) −x+4/3, −y+2/3, −z+5/3; (iv) −y+1, x−y, z; (v) −x+y+1, −x+1, z; (vi) −x+1/3, −y−1/3, −z+5/3; (vii) x+1, y+1, z; (viii) x, y+1, z; (ix) −x+4/3, −y−1/3, −z+5/3; (x) −x+5/3, −y+4/3, −z+4/3; (xi) x−y+2/3, x+1/3, −z+4/3; (xii) −x+y, −x, z; (xiii) x, y−1, z. |
Hydrogen-bond geometry (Å, º) top
| D—H···A | D—H | H···A | D···A | D—H···A |
| O3—H1···O1iii | 0.81 (2) | 1.79 (2) | 2.596 (7) | 180.00 |
| Symmetry code: (iii) −x+4/3, −y+2/3, −z+5/3. |
Unit-cell parameters of some
M1[M23(XO4)2(OH)3] compounds
(M1 = Ba, Pb; M2 = Co, Cu; X = V, As) top| Compound | a (Å) | b (Å) | c (Å) | α (°) | β (°) | γ (°) | V (Å3) | Reference |
| Ba[Co3(VO4)2(OH)2] | 5.9210 (8) | 5.9210 (8) | 21.016 (4) | 90 | 90 | 120 | 638.07 (18) | This work |
| Ba[Cu3(VO4)2(OH)2] | 10.270 (2) | 5.9110 (10) | 7.711 (2) | 90 | 116.42 (3) | 90 | 419.21 (15) | Ma et al. (1991) |
| Transformed cell# | 5.911 | 5.925 | 20.7171 | 90.06 | 90 | 119.92 | 628.8 | |
| Pb[Cu3(AsO4)2(OH)2] | 10.147 (2) | 5.8920 (10) | 14.081 (2) | 90 | 106.050 (10) | 90 | 809.0 (2) | Ghose & Wan (1979) |
| Transformed cell* | 5.892 | 5.867 | 20.313 | 91.87 | 90 | 120.14 | 606.8 | |
| Notes: (#) transformation matrix: 010/0.5 0.5 0/103;
(*) transformation matrix: 010/0.5 0.5 0/0.5 0 1.5. |

 Subscribe to Acta Crystallographica Section C: Structural Chemistry
Subscribe to Acta Crystallographica Section C: Structural Chemistry
The full text of this article is available to subscribers to the journal.
If you have already registered and are using a computer listed in your registration details, please email
support@iucr.org for assistance.

 Subscribe to Acta Crystallographica Section C: Structural Chemistry
Subscribe to Acta Crystallographica Section C: Structural Chemistry
 journal menu
journal menu








![[Figure 1]](http://journals.iucr.org/c/issues/2013/02/00/bi3052/bi3052fig1thm.gif)
![[Figure 2]](http://journals.iucr.org/c/issues/2013/02/00/bi3052/bi3052fig2thm.gif)
![[Figure 3]](http://journals.iucr.org/c/issues/2013/02/00/bi3052/bi3052fig3thm.gif)
![[Figure 4]](http://journals.iucr.org/c/issues/2013/02/00/bi3052/bi3052fig4thm.gif)

 Subscribe to Acta Crystallographica Section C: Structural Chemistry
Subscribe to Acta Crystallographica Section C: Structural Chemistry



There are many reports of divalent metal vanadates synthesized by high-temperature solid-state reactions (Chen et al., 2004; Azdouz et al.., 2010; Huang et al., 2012, and references therein). Hydrothermal methods have also proved to be effective for the synthesis of new vanadium compounds (Đorđević & Karanović, 2008, 2010, and references therein). Controlling the products of hydrothermal syntheses is often difficult because of their high sensitivity to specific reaction conditions. However, hydrothermal syntheses often result in well developed single crystals. An ongoing study concerning the low-temperature hydrothermal synthesis, crystallography and properties of arsenate and vanadate(V) compounds in the system M1O–M2O–X2O5–H2O (M1 = Sr, Cd, Ba, Bi, Hg; M2 = Mg, Mn, Fe, Co, Ni, Cu, Zn; X = As, V) has yielded a large number of new M12+–(H), M22+–(H) and M1–M2–(H) arsenates and vanadates (Đorđević, 2011; Đorđević & Karanović, 2008, 2010; Weil et al., 2009; Đorđević, Karanović & Tillmanns, 2008; Đorđević, Stojanović & Karanović, 2010; Stojanović et al., 2012, and references therein) that have been characterized structurally and, in part, by spectroscopic techniques. These compounds often form anionic frameworks built from MO6 octahedra and XO4 tetrahedra, with An+ cations as counterions.
We report here the hydrothermal synthesis and crystal structure of the new barium di-µ-hydroxydo-di-µ-vanadato-tricobaltate(II), Ba[Co3(VO4)2(OH)2]. In contrast with the topologically identical structures of vesignieite, Ba[Cu3(VO4)2(OH)2] (Zhesheng et al., 1991), and bayldonite, Pb[Cu3(AsO4)2(OH)2] (Ghose & Wan, 1979), which are monoclinic, Ba[Co3(VO4)2(OH)2] crystallizes in the rhombohedral space group R3m. Accordingly, it forms a structurally perfect Kagomé compound. It is well known that crystal structures containing layers with a Kagomé lattice arrangement display interesting physical properties connected with geometrically frustrated magnetism (Valldor et al., 2009; Caignaert et al., 2009; Olariu et al., 2008). Unfortunately, however, the quality and size of the Ba[Co3(VO4)2(OH)2] single crystals obtained in this work have not yet permitted further measurements of the physical properties.
In all three already mentioned M1[M23(XO4)2(OH)2] compounds (M1 = Ba2+, Pb2+; M2 = Co2+, Cu2+; X = V, As), a layered crystal structure has been found. There are two types of regularly alternating layers parallel to the (001) planes, viz. octahedral M23O6(OH)2 Kagomé layers separated by layers of XO4 tetrahedra and M1 cations in similar coordination environments (Fig. 1). In Ba[Co3(VO4)2(OH)2], the Co3O6(OH)2 layers of CoO4(OH)2 octahedra, with Co2+ and O22- ions in a two-dimensional Kagomé network, alternate along the c axis with barium vanadate anticuboctahedral–tetrahedral layers (Figs. 1 and 2). In the octahedral layers, six-membered octahedral rings are formed by edge-sharing of adjacent tetragonally shortened CoO4(OH)2 octahedra (Table 1 and Fig. 2). The Co2+ cation is surrounded by four equatorial oxide ligands [four symmetry equivalents of atom O2, with Co1—O2 = 2.161 (2) Å] and two axial hydroxide ligands [two symmetry equivalents of group O3H1, with Co1—O3 = 1.9449 (19) Å; Table 1 and Figs. 2 and 3]. The oxide ligands bridge between Co2+ centres to form an ideal Kagomé network composed of Co1O22 triangles (Fig. 4a). The distance between two Co2+ cation is 2.9605 (4) Å.
In the comparable monoclinic Ba[Cu3(VO4)2(OH)2] structure (Zhesheng et al., 1991), there are two crystallographically distinct positions for Cu and four for O, while in Pb[Cu3(AsO4)2(OH)2] (Ghose & Wan, 1979) there are three positions for Cu and five for O, due to the lower symmetry. Although in Ba[Cu3(VO4)2(OH)2] there are two Jahn–Teller-distorted Cu2+ cations in the Kagomé plane, the distortion of the CuO2 triangle from a regular geometry is negligible [2.9624 (5) Å for Cu1—Cu2 and 2.9555 (5) Å for Cu2—Cu2]. In Pb[Cu3(AsO4)2(OH)2], with three Cu sites, the distortion of the CuO2 triangle is somewhat larger, with the distances between two Cu 2+ cations being 2.9460 (5) Å for Cu1—Cu2, 2.9334 (4) Å for Cu1—Cu3 and 2.9334 (5) Å for Cu2—Cu3 (Fig. 4b).
In Ba[Co3(VO4)2(OH)2], the BaO12 polyhedron with Ba2+ at Wyckoff position 3b (0, 0, 1/2) (site symmetry 3m) is an anticuboctahedron with six shorter [2.824 (3) Å] and six longer [3.4186 (5) Å] distances (Table 1). The average Ba—O distance of 3.121 Å is slightly longer than the average Ba—O distance of 3.093 Å in Ba[Cu3(VO4)2(OH)2]. Around the smaller Pb2+ cation in Pb[Cu3(AsO4)2(OH)2], only eight O atoms are in the first coordination sphere, making a square antiprism with an average Pb—O distance of 2.721 Å. The next four Pb—O bonds, which are longer than 3.5 Å, contribute collectively to the bond-valence sum [Standard reference?] by ~10%. The differences in coordination for the M1 atoms are a consequence of the cation sizes and small shifts of the O atoms, caused by different orientations of the XO4 tetrahedra (Fig. 5).
The mutually isolated slightly-distorted VO4 tetrahedra have one short [1.661 (6) Å] and three longer [1.745 (3) Å] bonds (V—Oave = 1.724 Å). The short V—O bond involves atom O1, which is further bonded to three adjacent Ba2+ cations at longer distances and acts as the acceptor of a strong hydrogen bond (Table 2). The longer V—O bonds involve atom O2, which is further bonded to one Ba2+ cation at a shorter distance, and to two Co2+ cations. A similar distortion of the VO4 tetrahedra was found in Ba[Cu3(VO4)2(OH)2], where all V—O bonds are, in general, slightly shorter and range from 1.631 (6) to 1.7393 (3) Å (V—Oave = 1.712 Å). In the AsO4 tetrahedra of Pb[Cu3(AsO4)2(OH)2], there are two shorter and two longer bonds, which vary from 1.6646 (2) to 1.7229 (2) Å, and the average As—O distance (1.695 Å) is, as expected, shorter than V—Oave.
Atom H1 is localized near O3, which is further bonded to the three neighbouring Co2+ cations at shorter distances. Bond-valence sums for all atoms, calculated using the parameters of Brese & O'Keeffe (1991), give 1.68 v.u. (valence units) for Ba1, 2.14 v.u. for Co1 and 4.98 v.u. for V1. These values suggest that the Ba2+ cation is underbonded in its 12-coordinate site, while Co is slightly overbonded. Considering the contribution of the non-H atoms only, the bond-valence summations for the O atoms are 1.61 (O1), 1.97 (O2), and 1.52 v.u. (O3). Taking into account that atom O3 is the single donor of a strong hydrogen bond towards atom O1, the bond valences are well balanced (Table 2).
The present results show that Ba[Co3(VO4)2(OH)2] is not structurally identical to Ba[Cu3(VO4)2(OH)2] and Pb[Cu3(AsO4)2(OH)2]. The main difference is the higher rhombohedral symmetry. Thus, the Kagomé network in Ba[Co3(VO4)2(OH)2] is regular and closely similar, but not identical to, those in Ba[Cu3(VO4)2(OH)2] and Pb[Cu3(AsO4)2(OH)2]. Since Ba2+ is larger than Pb2+, Ba2+ is bonded to 12 O atoms, while Pb2+ is bonded only to eight. In order to compare the unit-cell dimensions, the monoclinic unit cells were transformed to the corresponding rhombohedral cells (Table 3). The resulting cells are almost identical for Ba[Co3(VO4)2(OH)2] and Ba[Cu3(VO4)2(OH)2], and, as expected, slightly smaller for Pb[Cu3(AsO4)2(OH)2].
In the literature, there are several compounds with Co atoms in the sites of the Kagomé lattice, with Ba atoms incorporated in the adjacent layers. Isostructural rhombohedral mixed-valence Ba2Co4ClO7 and Ba2Co4BrO7 (Kauffmann et al., 2007) have hole-filled Kagomé layers that consist of edge-sharing octahedra. Co3+ cations are positioned in the octahedral sites of the Kagomé lattice, whereas Co2+ cations are situated at the centres of the hexagons formed by the Kagomé lattice, i.e. they occupy the Kagomé holes. Layers built up from corner-sharing CoO6 octahedra, CoO4 tetrahedra and Ba2+ cations sandwich the Kagomé layers to form [Ba2Co8O14]2- blocks. Similar blocks are found in Ba2Co9O14 and Ba3Co10O17 (Sun et al., 2006; Ehora et al., 2007). It is also interesting to note the jarosite-type compounds, with the general formula AM3(SO4)2(OH)6 (A = Na, K, Rb, Tl; M = Fe3+, Cr3+, V3+), which adopt the same space group as Ba[Co3(VO4)2(OH)2] and also belong to the class of Kagomé compounds. One member of this group is NaV3(SO4)2(OD)6 jarosite, with V3+ cations in the positions of the Kagomé lattice (Grohol et al., 2003). In contrast with the linking role of the VO4 tetrahedra in Ba[Co3(VO4)2(OH)2], where V is in the +5 oxidation state, V can create the Kagomé lattice in jarosite as a +3 cation.
The Ba[Co3(VO4)2(OH)2] structure is also related to the rhombohedral (space group P31c) and orthorhombic (space group Pbn21) mixed-valence compound YbBaCo4O7 (Huq et al., 2006). YbBaCo4O7 is one member of the class of compounds with general formula RBaCo4O7, where R is Y, Tb, Dy, Ho, Er, Tm, Yb or Lu (Khalyavin et al., 2009, and references therein), better known as `114' oxides. These structures are characterized by Kagomé layers of CoO4 tetrahedra. There are two symmetry-independent CoO4 tetrahedra, one forming the Kagomé layers and another linking these layers along the c axis. These tetrahedra appear in a 1:3 ratio, which is in accordance with the nominal stoichiometry YbBaCo3+Co2+3O7. Similar structures are found for the rhombohedral (space group P31c) YBaAlCo3O7 (Valldor et al., 2008) and orthorhombic (space group Pbn21) CaBaCo4O7 (Caignaert et al., 2009).
Although all mentioned compounds have Kagomé layers, it is interesting to note the diverse role of Co: it can be Co2+, Co3+ or mixed valence, and in an octahedral or a tetrahedral coordination. In addition, the composition and geometry of the layers which sandwich the Kagomé layers and incorporate Ba2+ cations can be very different. Ba[Co3(VO4)2(OH)2] is a specific example of an ideal Kagomé compound, having a structure which consists of 1:1 ordered stacking of novel barium vanadate anticuboctahedral–tetrahedral layers and Kagomé layers of tetragonally shortened CoO4(OH)2 octahedra.